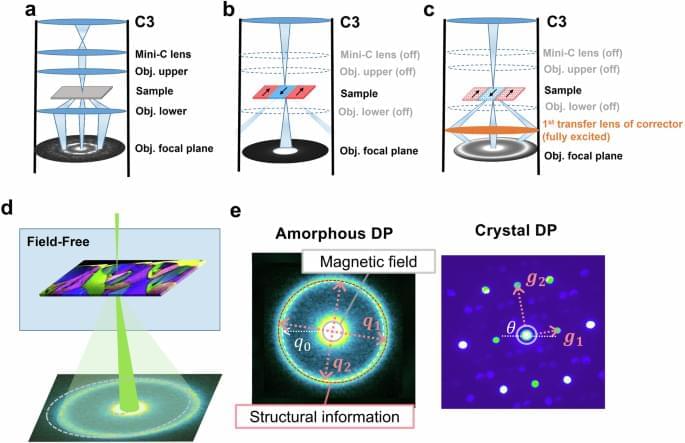
The authors present an approach to simultaneously map local magnetization, strain, atomic structure at nanoscale. It provides direct visualization of strainmagnetic coupling in ferromagnetic materials, opening avenues for studying nanomagnetism.
Large-angle Lorentz Four-dimensional scanning transmission electron microscopy for simultaneous local magnetization, strain and structure mapping
Posted in mapping, nanotechnology | Leave a Comment on Large-angle Lorentz Four-dimensional scanning transmission electron microscopy for simultaneous local magnetization, strain and structure mapping