Blue zones, places home to an unusual number of centenarians, are looked to for their secrets to living healthier lives – but are they even real?
Category: life extension
Is Chief Executive Officer and Managing Director of Cynata Therapeutics (https://cynata.com/), a stem cell and regenerative medicine company that is known for its proprietary Cymerus platform, for the scalable and consistent production of mesenchymal stem cell (MSC)-based therapies.
Unlike traditional MSC therapies that rely on multiple donors, the Cymerus manufacturing process ensures that cells for therapeutic use can be produced in virtually limitless quantities from a single donor – making the opportunities endless and attractive from a manufacturing standpoint. The company has completed Phase I studies for Graft vs Host disease & Diabetic Foot Ulcers and have a number of Phase II, and even have a Phase III clinical trial, in progress.
Dr. Kelly has over 20 years’ experience in biopharmaceutical research and development, including almost 15 years focused on the development of mesenchymal stem cell (MSC) based therapies. He joined Cynata in March 2014, initially as Vice President, Product Development, then Chief Operating Officer from May 2019, and since July 2023 has been CEO & MD. At Cynata, he has overseen all stages of the development of the Cymerus induced pluripotent stem cell (iPSC)-derived MSC technology, including the first completed clinical trial of any iPSC-derived product worldwide.
Dr. Kelly previously held positions at Biota Pharmaceuticals, Mesoblast Limited, Kendle International, Amgen and AstraZeneca.
Dr. Kelly holds a Masters in Pharmacy degree from the Robert Gordon University, Aberdeen, a Ph.D. in Pharmaceutical Sciences from Strathclyde University, Glasgow, and he is a Graduate of the Australian Institute of Company Directors (AICD), Melbourne. He is a member of the International Society for Cell and Gene Therapy (ISCT), the International Society for Stem Cell Research (ISSCR), the Royal Pharmaceutical Society and the AICD.
Dr. Kelly also serves on the ISCT Asia-Pacific Industry Committee, the ISSCR Best Practices Working Group for the Development of PSC-Derived Therapies and the Industry Interface Committee of the Center for Commercialization of Regenerative Medicine (CCRM) Australia.
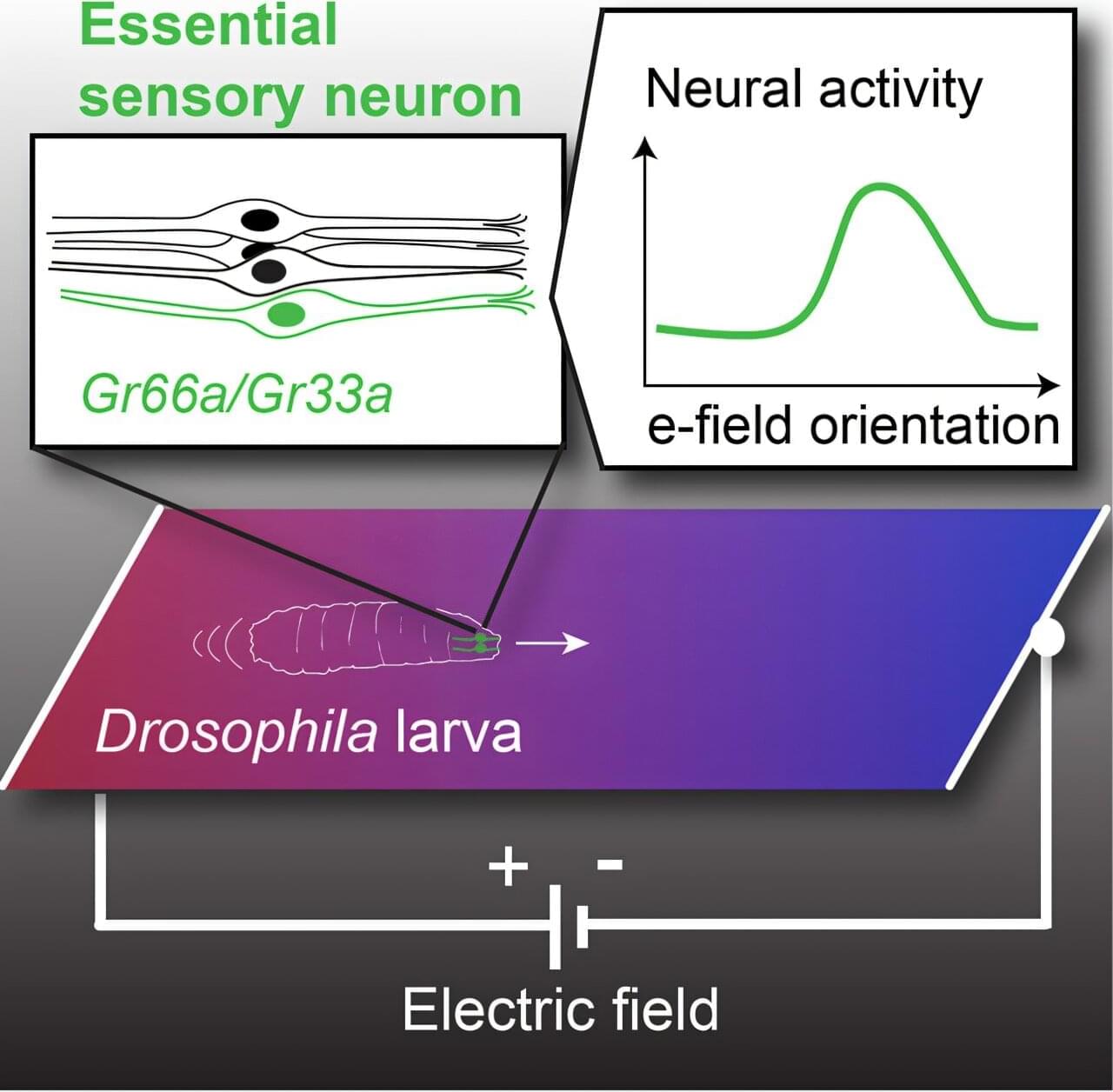
While it may be an unfamiliar sensation to humans, electroreception is relatively commonplace in the animal kingdom. Sharks, bees and even the platypus all share this ability to detect electric fields in their environment.
Scientists at UC Santa Barbara have just added fruit flies to that list. A team of researchers led by Matthieu Louis found that fruit fly larvae can sense electric fields and navigate toward the negative electric potential using a small set of sensory neurons in their head.
The findings, published in Current Biology, present an immense opportunity. Fruit flies are arguably the most commonly used experimental animals, the basis for studies in fields as disparate as genetics, neurobiology and aging. Uncovering electroreception in fruit flies opens new avenues of research into the basis of this sense and could even lead to new techniques in bioengineering.
At 102, Mike attributes his longevity and active lifestyle to a macrobiotic diet and physical discipline. Diagnosed with cancer at 69, he turned to a whole, plant-based diet, which he believes, along with regular exercise and minimalistic living, reversed his condition and maintains his vitality.
Damage to the mitochondria, the “power plants” of the cells, contributes to many diseases. Researchers from Heinrich Heine University Düsseldorf (HHU) and the University of Cologne led by HHU professor of medicine Dr David Pla-Martín, now describe in the scientific journal Science Advances how cells with defective mitochondria activate a special recycling system to eliminate damaged genetic material.
Damage to the genetic material of mitochondria – the mitochondrial DNA or mtDNA for short – can lead to diseases such as Parkinson’s, Alzheimer’s, amyotrophic lateral sclerosis (ALS), cardiovascular diseases and type 2 diabetes. Such damage also speeds up the ageing process. However, the cells are normally capable of identifying such damage and reacting.
Damage to the genetic material of mitochondria—the mitochondrial DNA or mtDNA for short—can lead to diseases such as Parkinson’s, Alzheimer’s, amyotrophic lateral sclerosis (ALS), cardiovascular diseases and type 2 diabetes. Such damage also speeds up the aging process. However, the cells are normally capable of identifying such damage and reacting.
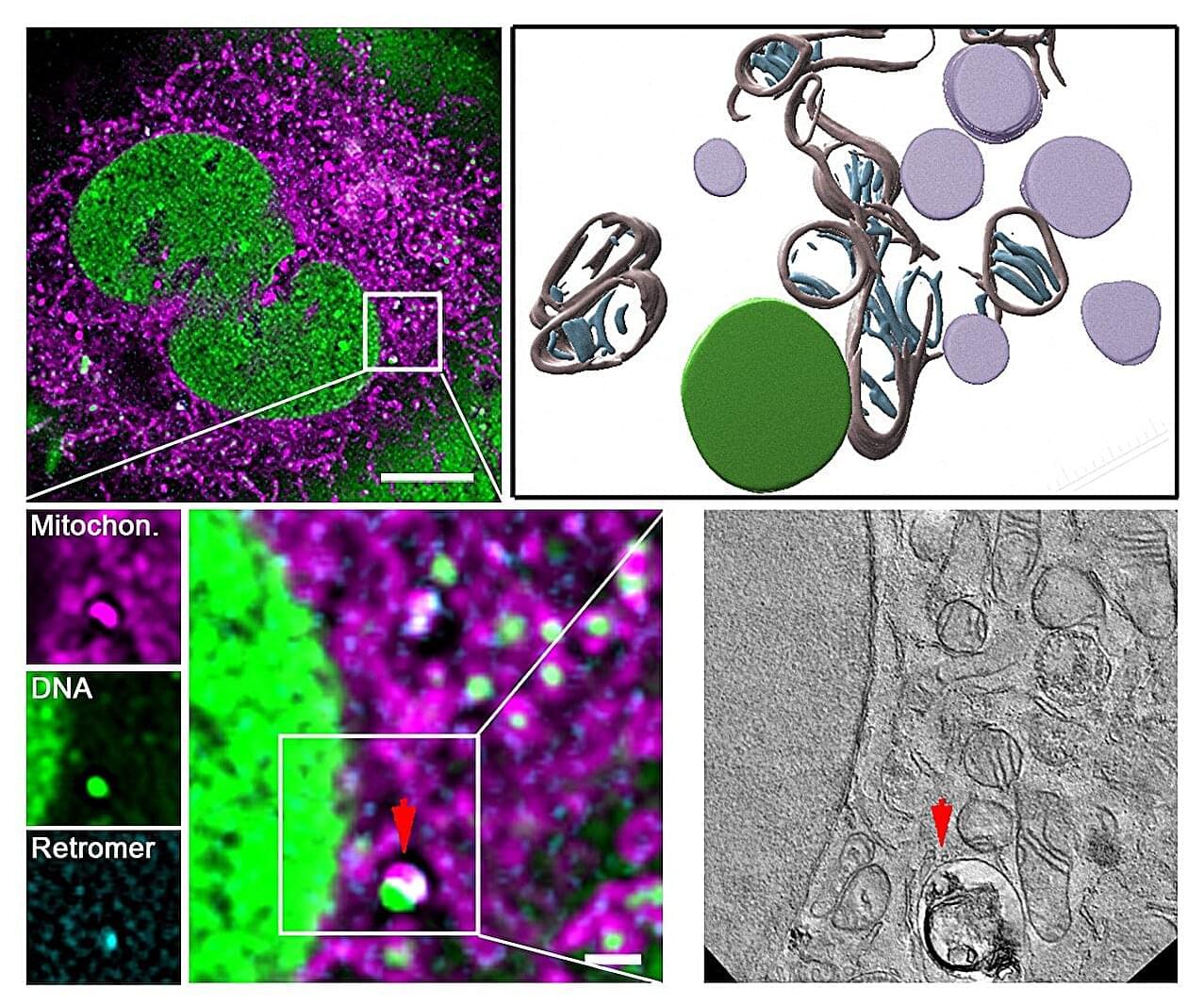
Scientists from University Hospital Düsseldorf and HHU have—in collaboration with the University of Cologne and the Center for Molecular Medicine Cologne (CMMC)—discovered a mechanism which protects and repairs the mitochondria. The research team, headed by Professor Pla-Martín from the Institute of Biochemistry and Molecular Biology I at HHU, has identified a specialized recycling system, which cells activate when they identify damage to the mtDNA.
According to the authors in Science Advances, this mechanism relies on a protein complex known as retromer and the lysosomes—cell organelles containing digestive enzymes. These special cellular compartments act like recycling centers, eliminating the damaged genetic material.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
We live longer and longer, and as we age, a lot of us develop a series of health issues and chronic diseases, including Chronic Obstructive Pulmonary Disease (COPD), which is found in around 600 million individuals globally. However, only half of them know they have the disease.
COPD patients often experience shortness of breath, persistent cough with mucus, wheezing and frequent respiratory infections, which can make everyday activities difficult.
Now a new study from the University of Copenhagen and Bispebjerg Hospital suggests that a form of vitamin B3 may be the key to improving quality of life for these patients.
“In the study, we show that nicotinamide riboside, also known as vitamin B3, can reduce lung inflammation in COPD patients,” says Associate Professor Morten Scheibye-Knudsen from the Center for Healthy Aging at the Department of Cellular and Molecular Medicine, University of Copenhagen, who has co-authored the new study.
A new study carried out by researchers at the University of Copenhagen and Bispebjerg Hospital shows that a form of vitamin B3 can reduce lung inflammation in COPD patients.
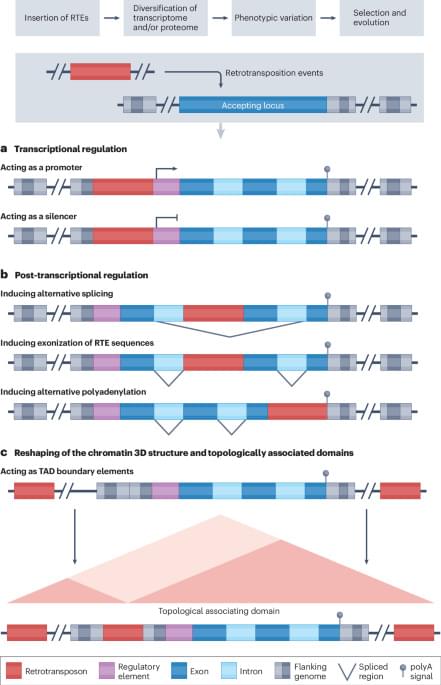
In this Review, Della Valle et al. discuss the role of retrotransposable elements (RTEs) in the onset and progression of ageing and ageing-related disease, including evidence that environmental stressors act through RTEs to shift the trajectory towards unhealthy ageing.
In two longitudinal cohorts followed for 30 years, the associations of eight different dietary patterns with healthy aging—encompassing cognitive, physical and mental health—were studied, identifying food constituents linked to greater or lesser odds of healthy aging.