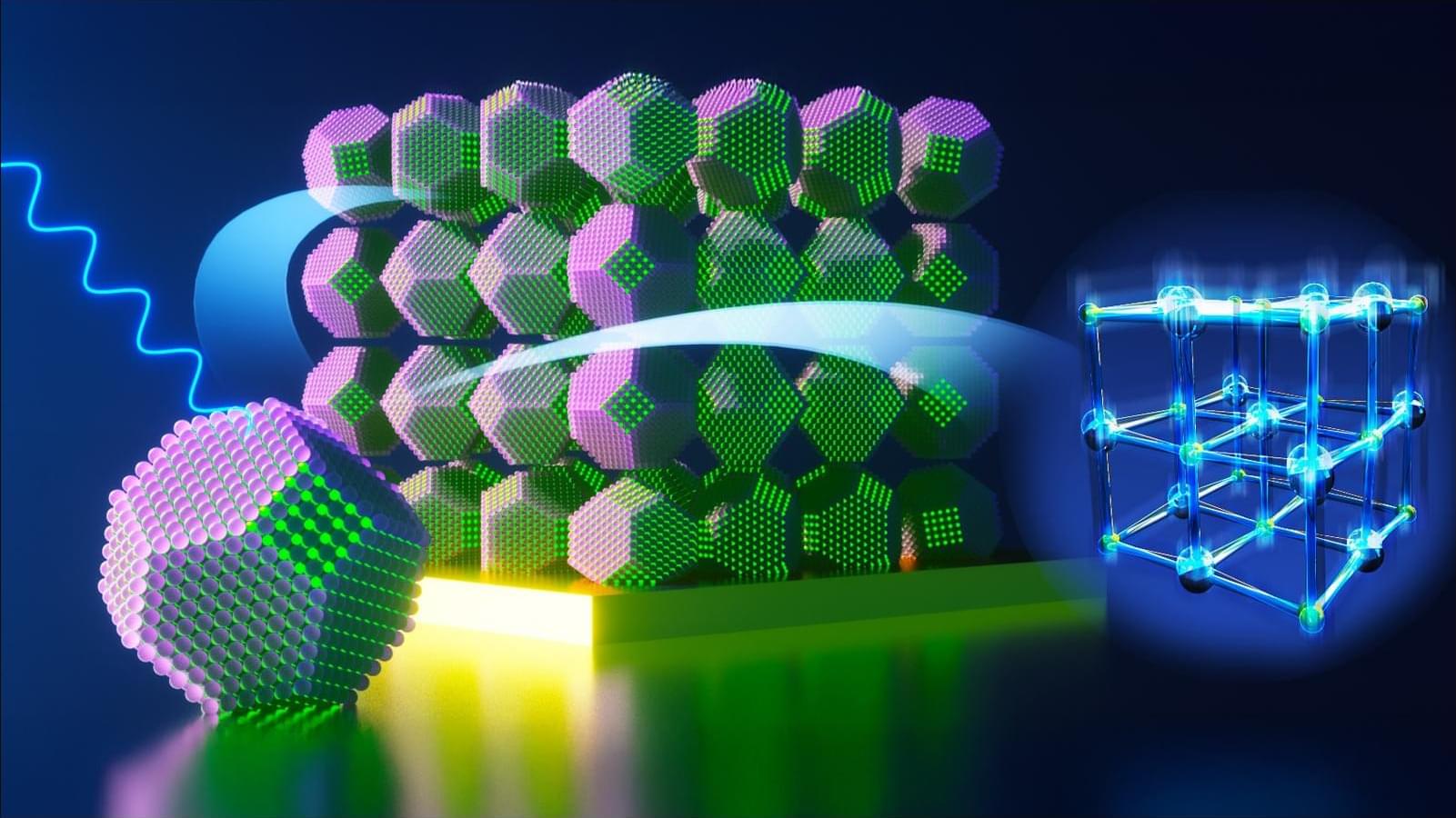
Breakthrough allows researchers to create materials with tailored properties, unlocking unprecedented control over their optical and electronic properties.
Category: materials
Titanium micro-particles in the oral mucosa around dental implants are common. This is shown in a new study from the University of Gothenburg and Uppsala University, which also identified 14 genes that may be affected by these particles.
Registry data indicate that about 5% of all adults in Sweden have dental implants —and potentially also titanium particles in the tissue surrounding the implants. According to the researchers, there is no reason for concern, but more knowledge is needed.
“Titanium is a well-studied material that has been used for decades. It is biocompatible and safe, but our findings show that we need to better understand what happens to the micro-particles over time. Do they remain in the tissue or spread elsewhere in the body?” says Tord Berglundh, senior professor of periodontology at Sahlgrenska Academy, University of Gothenburg.
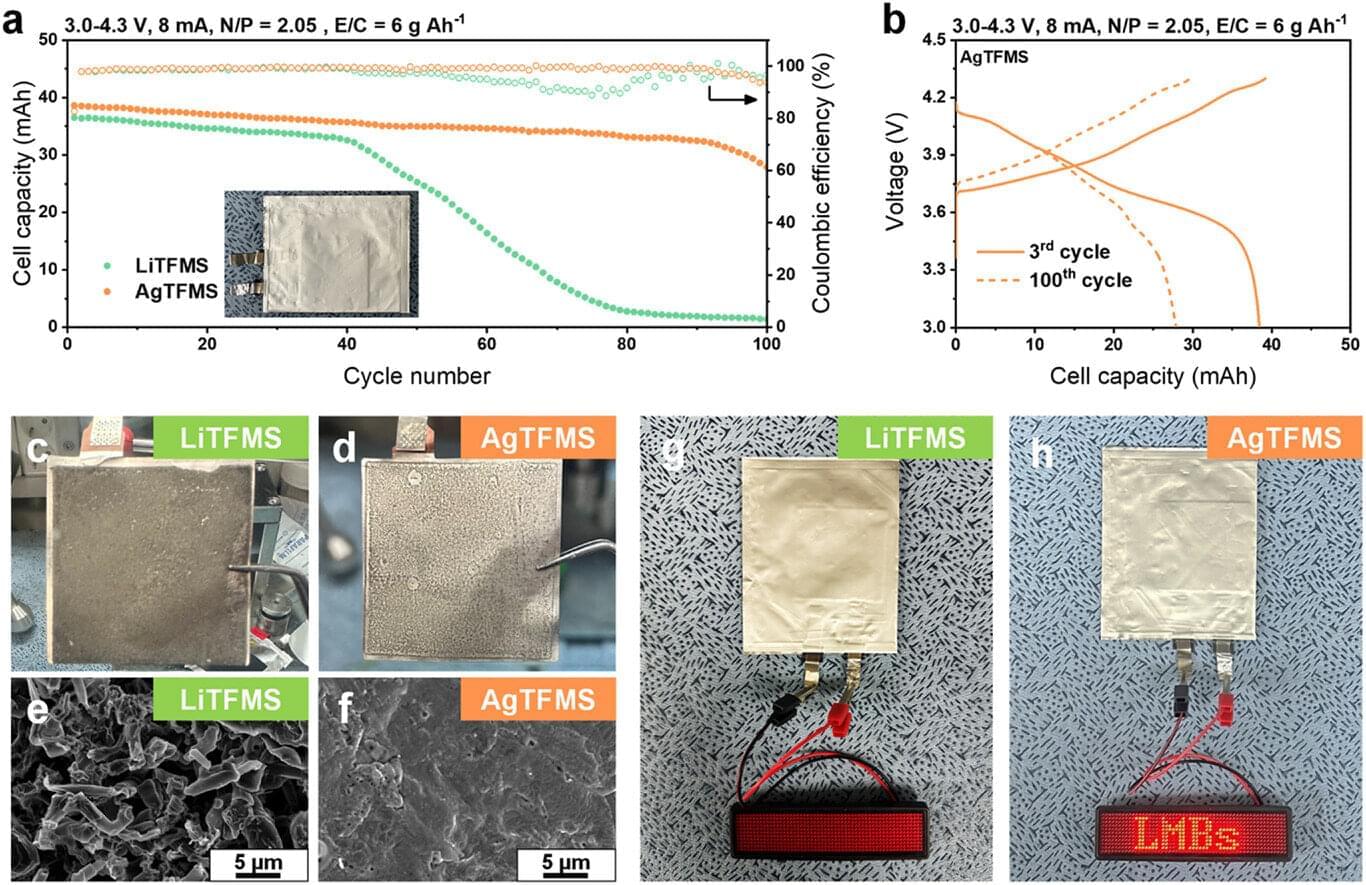
A research team has developed a technology that dramatically enhances the stability of ultra-thin metal anodes with a thickness of just 20μm. Led by Professor Yu Jong-sung from the Department of Energy Science and Engineering at DGIST, the team proposed a new method using electrolyte additives to address the issues of lifetime and safety that have hindered the commercialization of lithium metal batteries. The work is published in the journal Advanced Energy Materials.
Lithium metal anodes (3,860 mAh g⁻¹) have over 10 times the capacity of widely used graphite anodes (372 mAh g⁻¹) and feature a low standard reduction potential, making them promising candidates for next-generation anode materials. However, during charge-discharge cycles, lithium tends to grow in dendritic forms, causing short circuits and thermal runaway, which leads to lifetime and safety issues. Moreover, due to volume expansion, the solid electrolyte interphase (SEI) repeatedly degrades and reforms, leading to rapid electrolyte depletion.
The use of ultra-thin lithium metal with a thickness below 50μm is essential, especially for the commercialization of lithium metal batteries. However, such issues become more severe as thickness reduces. Accordingly, both academia and industry have focused on SEI engineering to enhance the stability of lithium metal anodes, among which SEI formation strategies using electrolyte additives have emerged as a simple yet effective approach.
University of Oregon chemists are bringing a greener way to make iron metal for steel production closer to reality, a step towards cleaning up an industry that’s one of the biggest contributors to carbon emissions worldwide. The research was published in ACS Energy Letters.
Last year, UO chemist Paul Kempler and his team reported a way to create iron with electrochemistry, using a series of chemical reactions that turn saltwater and iron oxide into pure iron metal.
In their latest work, they’ve optimized the starting materials for the process, identifying which kinds of iron oxides will make the chemical reactions the most cost-effective. That’s a key to making the process work at an industrial scale.
Request PDF | Degradable thermosets via orthogonal polymerizations of a single monomer | Crosslinked thermosets are highly durable materials, but overcoming their petrochemical origins and inability to be recycled poses a grand… | Find, read and cite all the research you need on ResearchGate
Arranging nonmagnetic atoms on the surface of an unconventional superconductor could induce a novel phenomenon called altermagnetic superconductivity.
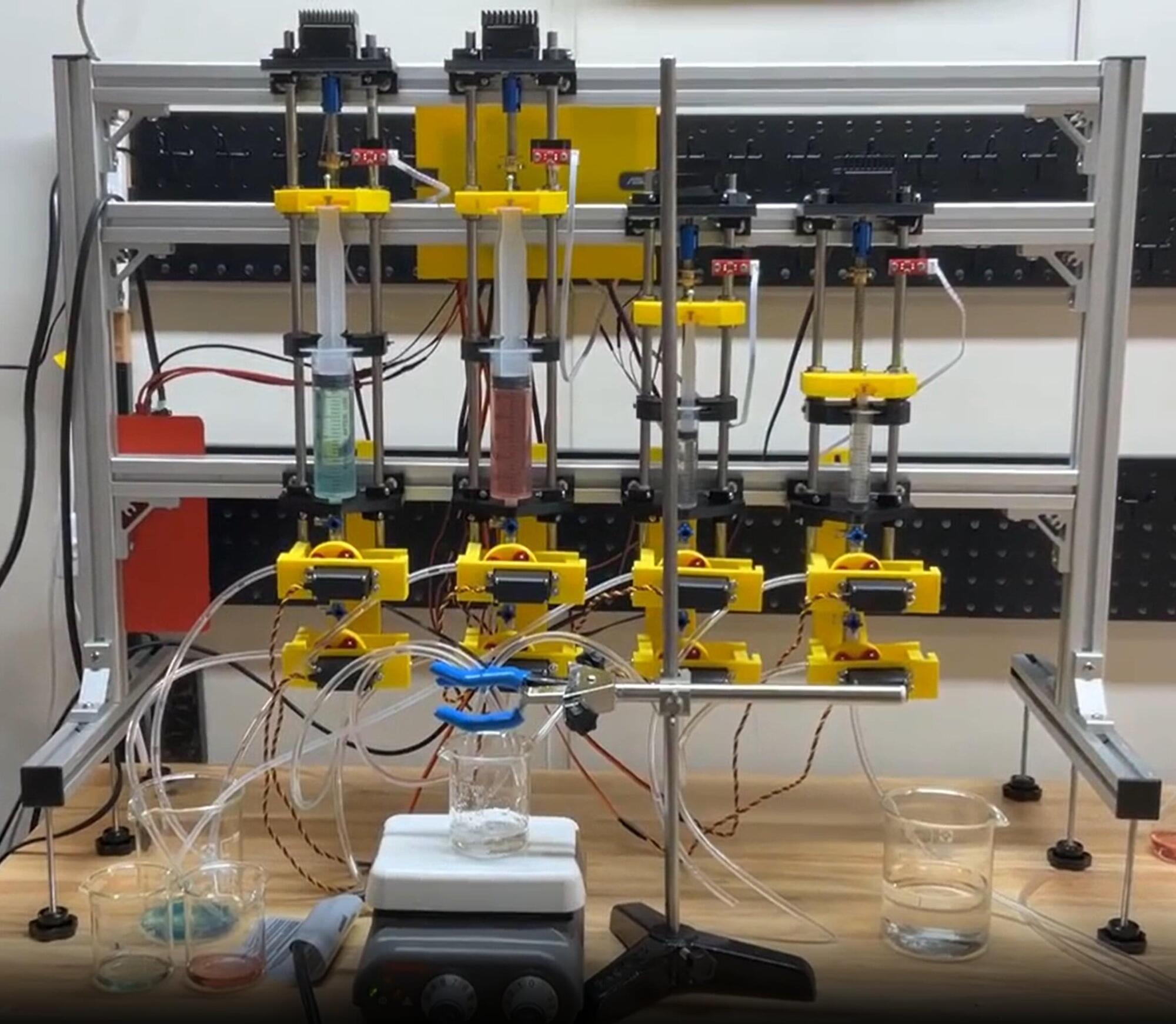
A team of researchers led by Professor Keisuke Takahashi at the Faculty of Science, Hokkaido University, have created FLUID (Flowing Liquid Utilizing Interactive Device), an open-source robotic system constructed using a 3D printer and off-the-shelf electronic components.
To demonstrate FLUID’s capabilities, the team used the robot to automate the co-precipitation of cobalt and nickel, creating binary materials with precision and efficiency.
“By adopting open source, utilizing a 3D printer, and taking advantage of commonly-available electronics, it became possible to construct a functional robot that is customized to a particular set of needs at a fraction of the costs typically associated with commercially-available robots,” said Mikael Kuwahara, the lead author of the study.
University of Warwick astronomers have discovered an extremely rare, high-mass, compact binary star system only ~150 light years away. These two stars are on a collision course to explode as a type 1a supernova, appearing 10 times brighter than the moon in the night sky.
Type 1a supernovae are a special class of cosmic explosion, famously used as “standard candles” to measure distances between Earth and their host galaxies. They occur when a white dwarf (the dense remnant core of a star) accumulates too much mass, is unable to withstand its own gravity, and explodes.
It has long been theoretically predicted that two orbiting white dwarfs are the cause of most type 1a supernova explosions. When in a close orbit, the heavier white dwarf of the pair gradually accumulates material from its partner, which leads to that star (or both stars) exploding.
For many years, physics studies focused on two main types of magnetism, namely ferromagnetism and antiferromagnetism. The first type entails the alignment of electron spins in the same direction, while the latter entails the alignment of electron spins in alternating, opposite directions.
Yet recent studies have discovered a new kind of magnetism, referred to as altermagnetism, which does not fit into either of the previously identified categories. Altermagnetism is characterized by the breaking of time-reversal symmetry (i.e., the symmetry of physical laws when time is reversed) and spin-split band structures, in materials that retain a zero net magnetization.
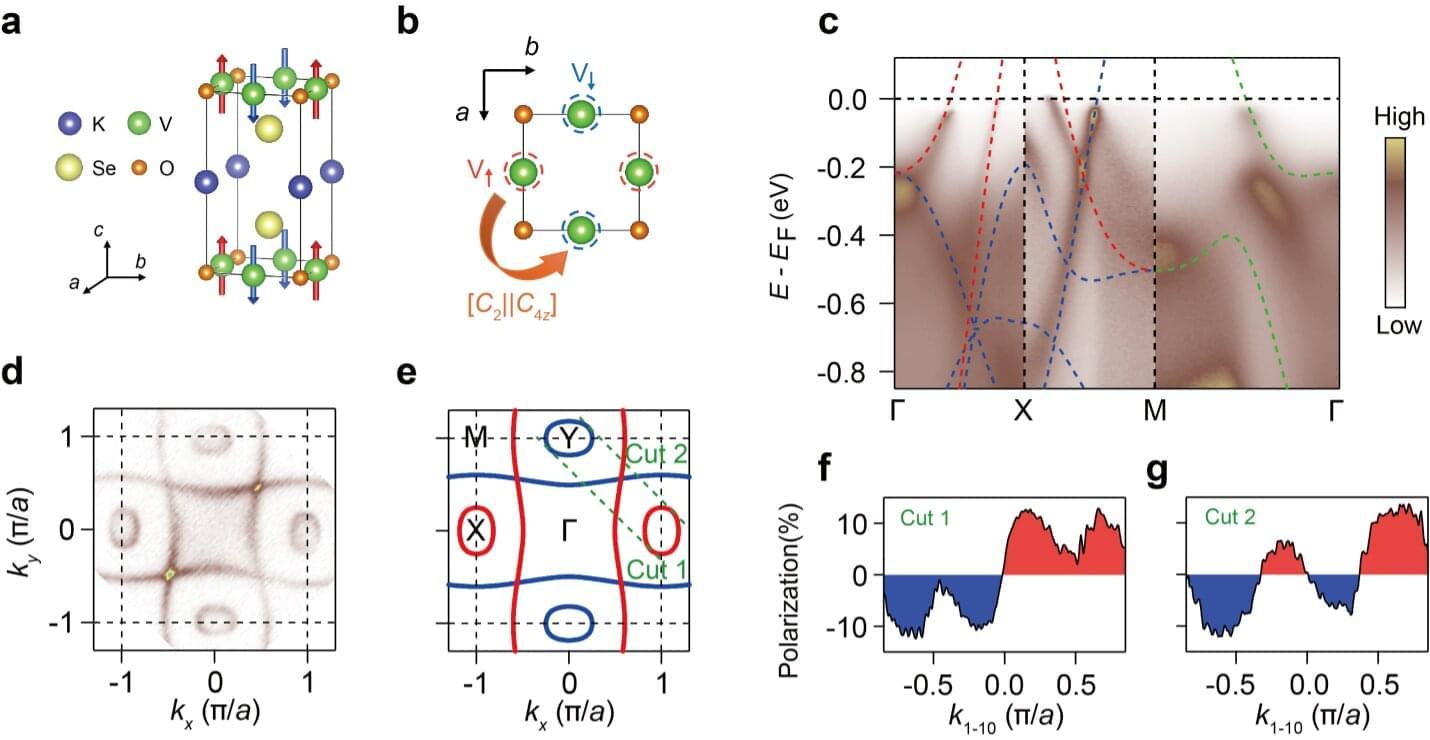
Researchers at the Chinese Academy of Sciences and other institutes in China recently uncovered a new material that exhibits altermagnetism at room temperature, namely KV2Se2O. Their findings, published in Nature Physics, highlight the promise of KV₂Se₂O both for the study of altermagnetism and for the development of spintronic devices.
Lacquers, paint, concrete—and even ketchup or orange juice: Suspensions are widespread in industry and everyday life. By a suspension, materials scientists mean a liquid in which tiny, insoluble solid particles are evenly distributed. If the concentration of particles in such a mixture is very high, phenomena can be observed that contradict our everyday understanding of a liquid. For example, these so-called non-Newtonian fluids suddenly become more viscous when a strong force acts upon them. For a brief moment, the liquid behaves like a solid.
This sudden thickening is caused by the particles present in the suspension. If the suspension is deformed, the particles have to rearrange themselves. From an energy perspective, it is more advantageous if they roll past each other whenever possible. It is only when this is no longer possible, e.g., because several particles become jammed, that they have to slide relative to each other. However, sliding requires much more force and thus the liquid feels macroscopically more viscous.
The interactions that occur on a microscopically small scale therefore affect the entire system and they determine how a suspension flows. To optimize the suspension and specifically influence its flow characteristics, scientists must therefore understand the magnitude of the frictional forces between the individual particles.