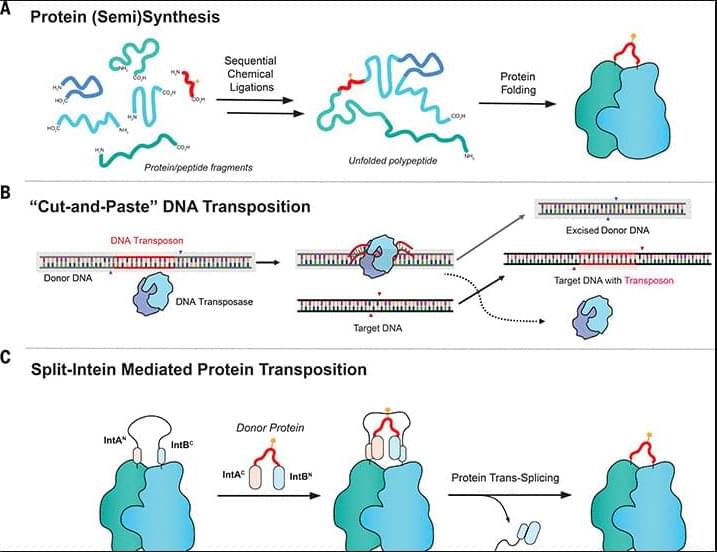
Protein engineering through the ligation of polypeptide fragments has proven enormously powerful for studying biochemical processes. In general, this strategy necessitates a final protein-folding step, constraining the types of systems amenable to the approach. Here, we report a method that allows internal regions of target proteins to be replaced in a single operation. Conceptually, our system is analogous to a DNA transposition reaction but uses orthogonal pairs of engineered split inteins to mediate the editing process. This “protein transposition” reaction is applied to several systems, including folded protein complexes, allowing the efficient introduction of a variety of noncoded elements. By carrying out a molecular “cut and paste” under native protein-folding conditions, our approach substantially expands the scope of protein semisynthesis.
Category: engineering
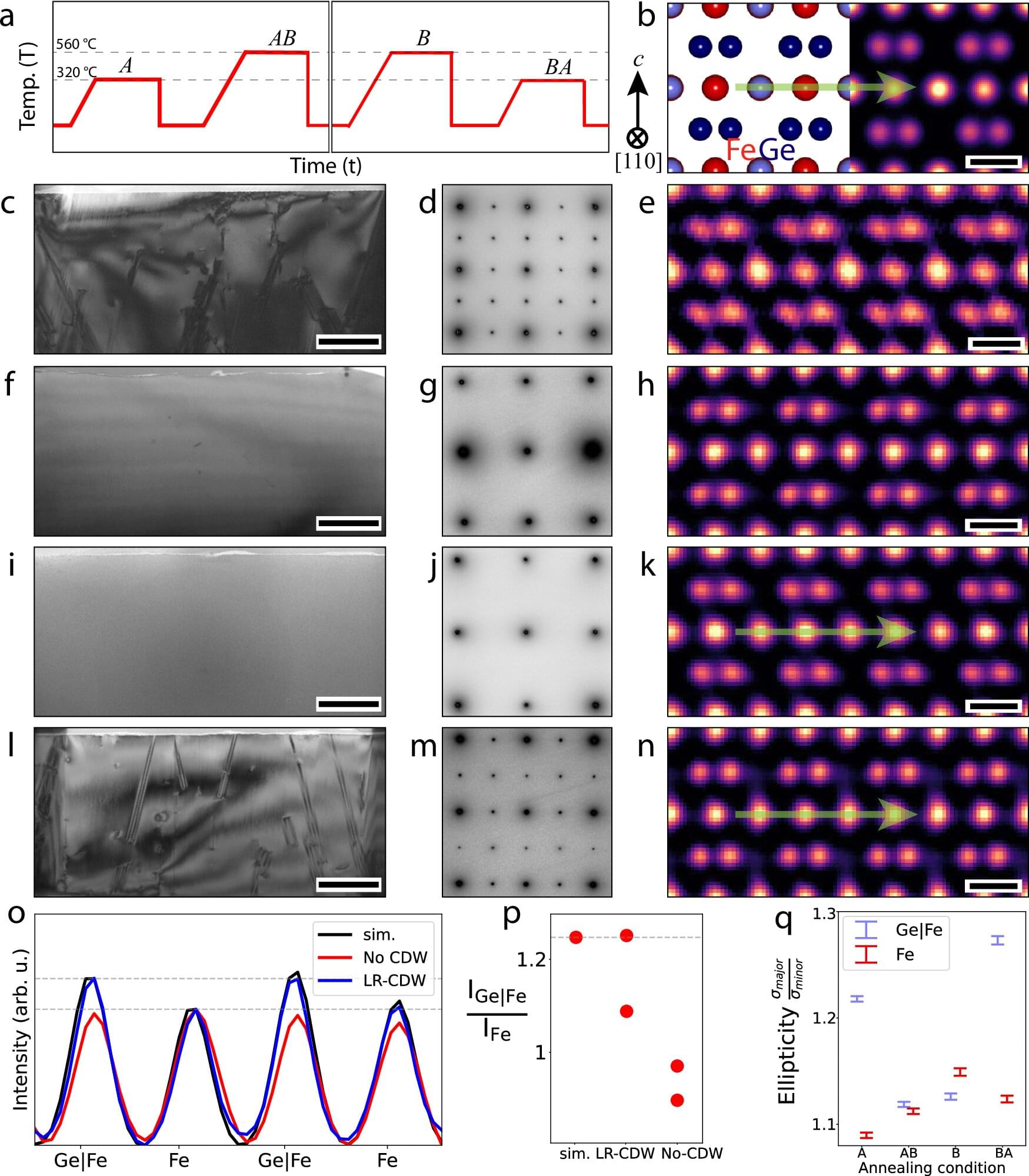
A new study published in Nature Communications April 7 could reshape the future of magnetic and electronic technology. Scientists at Rice University have discovered how a disappearing electronic pattern in a quantum material can be revived under specific thermal conditions. The finding opens new doors for customizable quantum materials and in-situ engineering, where devices are manufactured or manipulated directly at their point of use.
Led by Pengcheng Dai, the Sam and Helen Worden Professor of Physics and Astronomy, the researchers uncovered the cause behind a vanishing electronic phenomenon in a class of crystalline materials known as kagome lattice, a geometric arrangement of corner-sharing triangles named after a traditional Japanese basket pattern.
This discovery reveals how heating methods impact the presence of a charge density wave (CDW), a quantum pattern of electron arrangement, in the kagome metal iron germanide (FeGe). It also demonstrates how its reappearance enhances magnetic and electronic properties.
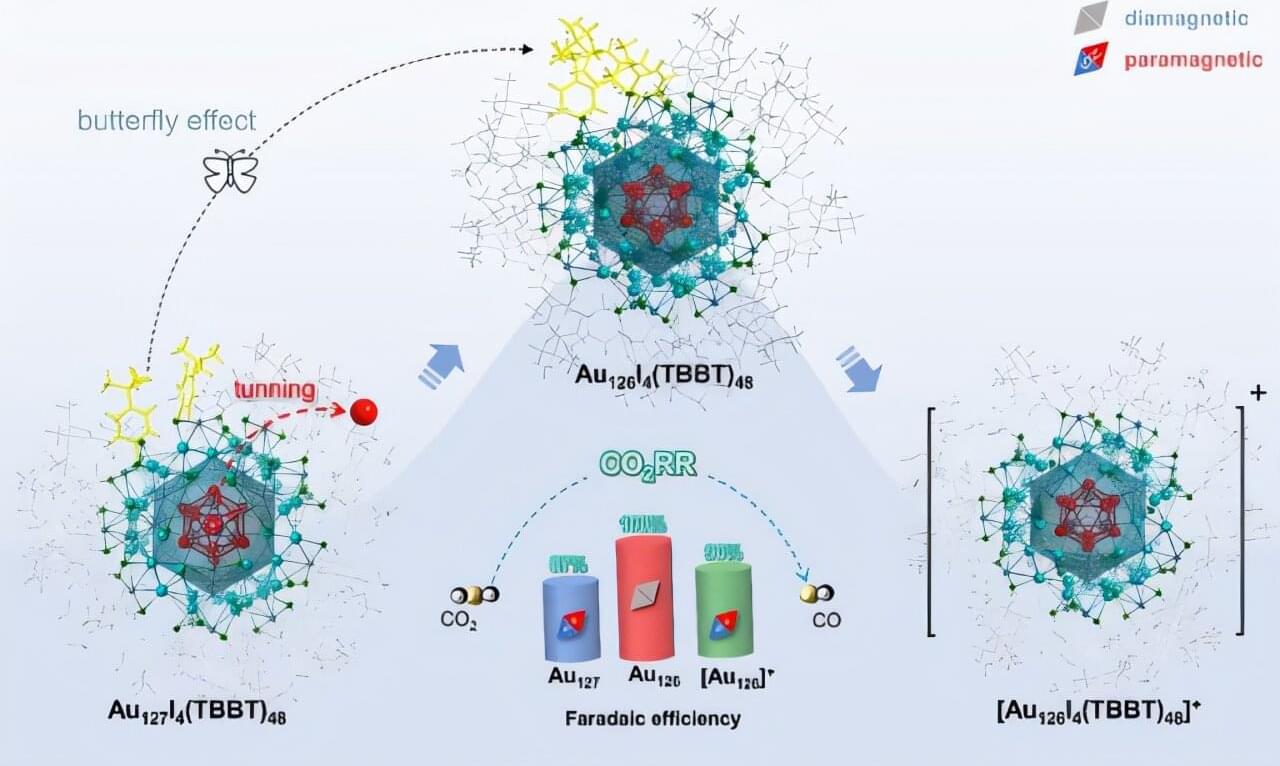
Recently, a team of researchers from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences (CAS) consecutively removed the innermost atom and the outermost electron of a gold nanoparticle—without disturbing its overall structure. This precise manipulation allowed them to probe how the magnetic spin of the material influences its catalytic activity.
The work, led by Prof. Wu Zhikun in collaboration with Prof. Yang from the Institute of Process Engineering, CAS and Prof. Tang from Chongqing University, was published in Science Advances.
Gold nanoclusters—tiny particles composed of from a few to hundreds of gold atoms —are ideal models for studying how atomic structure affects material properties. But tuning the structure of such clusters atom by atom, especially when they’re relatively large and complex, has long been a major challenge.
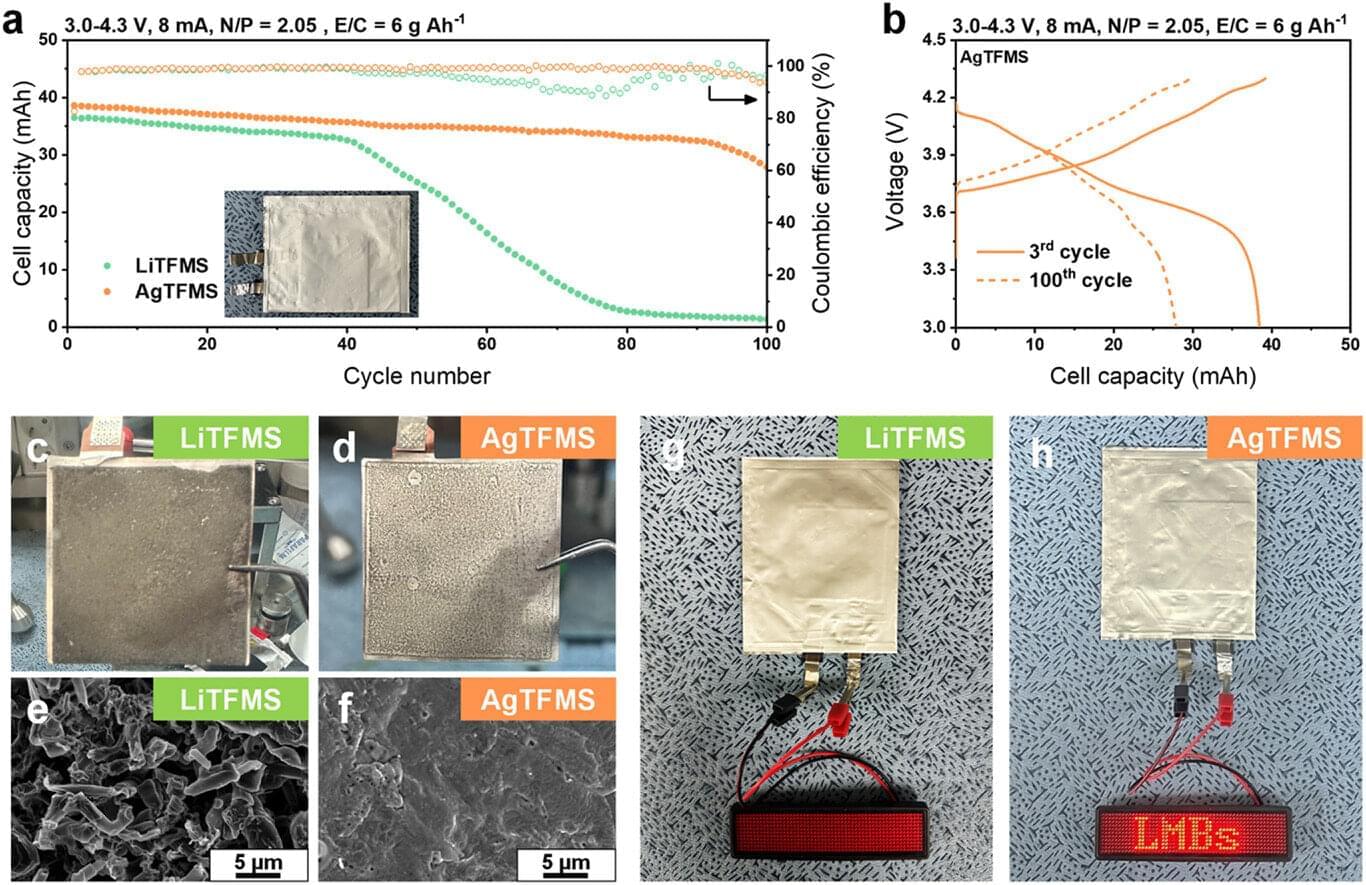
A research team has developed a technology that dramatically enhances the stability of ultra-thin metal anodes with a thickness of just 20μm. Led by Professor Yu Jong-sung from the Department of Energy Science and Engineering at DGIST, the team proposed a new method using electrolyte additives to address the issues of lifetime and safety that have hindered the commercialization of lithium metal batteries. The work is published in the journal Advanced Energy Materials.
Lithium metal anodes (3,860 mAh g⁻¹) have over 10 times the capacity of widely used graphite anodes (372 mAh g⁻¹) and feature a low standard reduction potential, making them promising candidates for next-generation anode materials. However, during charge-discharge cycles, lithium tends to grow in dendritic forms, causing short circuits and thermal runaway, which leads to lifetime and safety issues. Moreover, due to volume expansion, the solid electrolyte interphase (SEI) repeatedly degrades and reforms, leading to rapid electrolyte depletion.
The use of ultra-thin lithium metal with a thickness below 50μm is essential, especially for the commercialization of lithium metal batteries. However, such issues become more severe as thickness reduces. Accordingly, both academia and industry have focused on SEI engineering to enhance the stability of lithium metal anodes, among which SEI formation strategies using electrolyte additives have emerged as a simple yet effective approach.
The Korea Electrotechnology Research Institute (KERI) and the Korea Institute of Materials Science (KIMS) have jointly developed spray drying technology-based high-performance dry electrode manufacturing technology for the realization of high-capacity secondary batteries. The study is published in the Chemical Engineering Journal.
Secondary battery electrodes are made by mixing active materials that store electrical energy, conductive additives that help the flow of electricity, and binders which act as a kind of adhesive. There are two methods for mixing these materials: the wet process, which uses solvents, and the dry process, which mixes solid powders without solvents.
The dry process is considered more environmentally friendly than the wet process and has gained significant attention as a technology that can increase the energy density of secondary batteries. However, until now, there have been many limitations to achieving a uniform mixture of active materials, conductive additives, and binders in the dry process.
Modeling Path Effects Due to 3D Velocity Structure for Nonergodic Ground‐Motion Models: A Case Study Using Turkish Ground‐Motion Data
Posted in engineering | Leave a Comment on Modeling Path Effects Due to 3D Velocity Structure for Nonergodic Ground‐Motion Models: A Case Study Using Turkish Ground‐Motion Data
Ground motion models developed using global databases of ground motion rely on something called the ergodic assumption, which, for broad tectonic types, means that intensity measures for a given earthquake in a given region can be applied to any location in that region. A non-ergodic ground motion model instead takes into account how ground motion varies with source, site and/or path effects.
In a new study, a team from Georgia Tech, UC Berkeley, Orta Dogu Teknik Universitesi, and Pacific Gas and Electric Company assesses the performance of different path-effect models for developing non-ergodic ground motion models using a Turkish ground-motion database. For more about their findings, please visit the paper.
ABSTRACT. The objective of this study is to assess the performance of different path‐effect models for developing nonergodic ground motion models (GMMs) using a Turkish ground‐motion database. The cell‐specific attenuation approach is widely used to capture path effects in the formulation of nonergodic GMMs. However, this approach can mainly capture anelastic attenuation effects associated with the spatial variation of the quality factor, and it is limited in capturing 3D velocity structure effects, which may be, in particular, relevant for long‐period ground motions or short‐distance and short‐period ground motions. Recent efforts have introduced new models to incorporate 3D velocity structure effects; however, the assessment of these models in the context of instrumentally recorded ground motions is limited. This study assesses the performance of three path‐effects models for Türkiye. Specifically, we consider the cell‐specific attenuation approach and two additional models based on Gaussian processes but with a different parametrization on how they represent the spatial correlation of path effects. The results indicate that the models based on Gaussian processes outperform the cell‐specific approach for long‐period spectral accelerations and short‐period ground motions at short distances, offering significant aleatory standard deviation reductions. The differences between the Gaussian process‐based models are also discussed, highlighting how their parameterization is reflected in prediction patterns. This study contributes to the transition from ergodic to nonergodic approaches in performance‐based earthquake engineering.
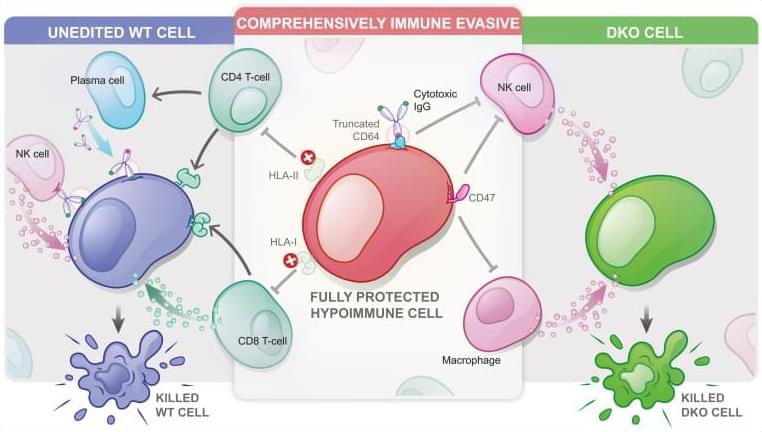
Allogeneic cell therapeutics are currently being developed to overcome manufacturing bottlenecks of autologous products but face allorejection as their biggest obstacle. This review analyzes the immunogenicity of allogeneic cell therapeutics, outlines engineering strategies for immune evasion, and summarizes recent milestone achievements.
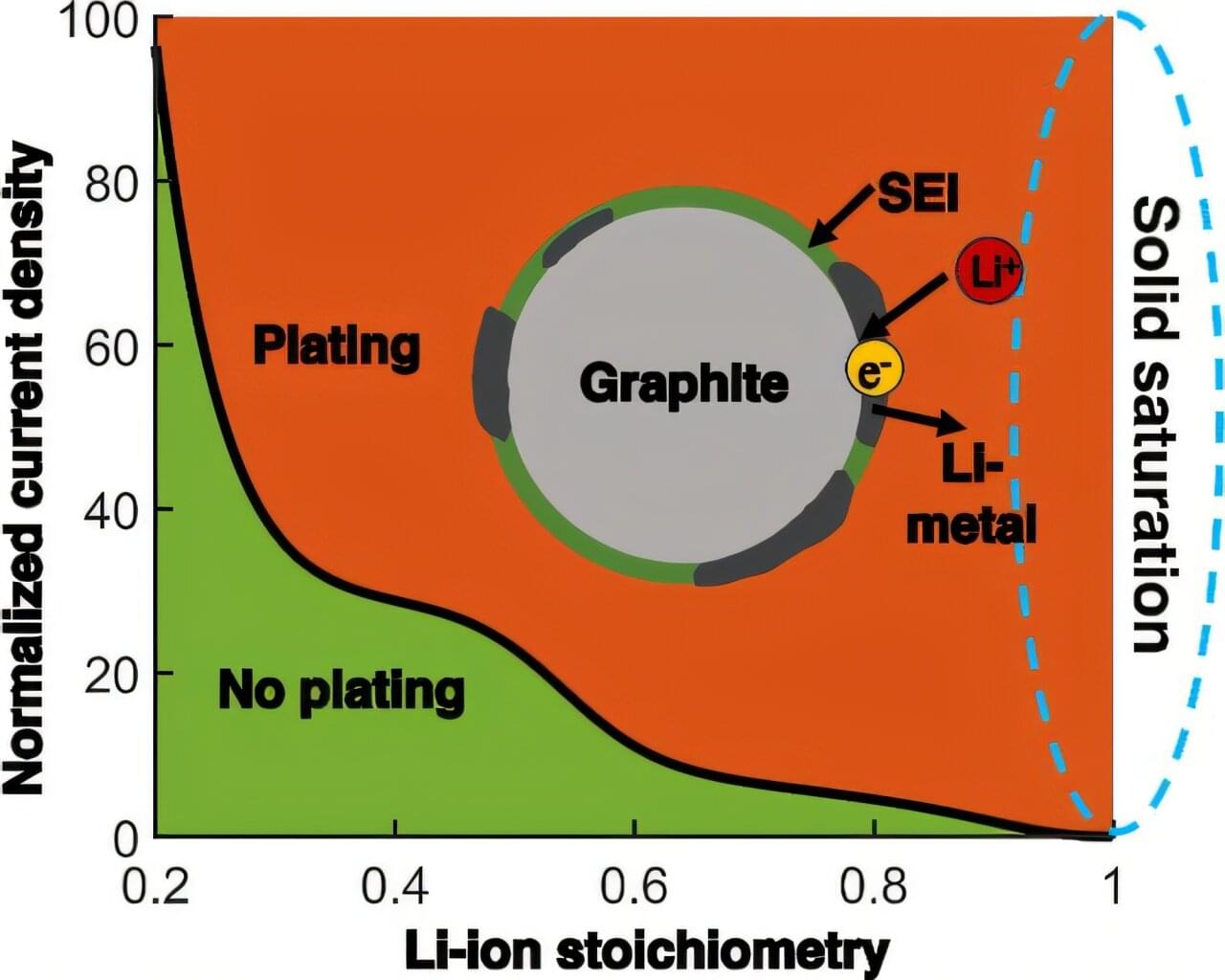
Fast-charging lithium-ion batteries are ubiquitous, powering everything from cellphones and laptops to electric vehicles. They’re also notorious for overheating or catching fire.
Now, with an innovative computational model, a University of Wisconsin–Madison mechanical engineer has gained new understanding of a phenomenon that causes lithium-ion batteries to fail.
Developed by Weiyu Li, an assistant professor of mechanical engineering at UW–Madison, the model explains lithium plating, in which fast charging triggers metallic lithium to build up on the surface of a battery’s anode, causing the battery to degrade faster or catch fire.
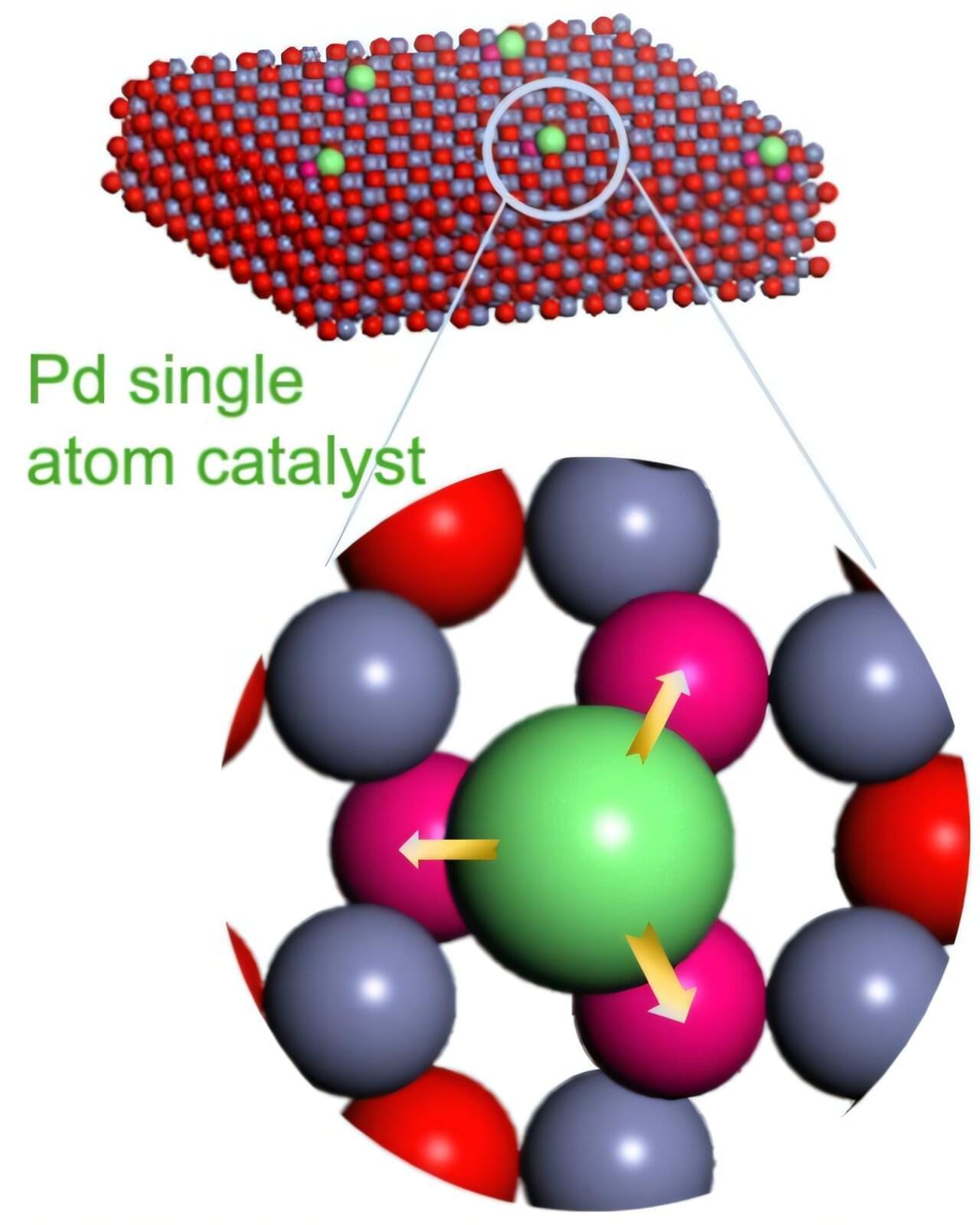
A chemical reaction that’s vital to a range of commercial and industrial goods may soon be initiated more effectively and less expensively thanks to a collaboration that included Oregon State University College of Engineering researchers.
The study, published in Nature, involves hydrogenation —adding the diatomic hydrogen molecule, H2, to other compounds.
“Hydrogenation is a critical and diverse reaction used to create food products, fuels, commodity chemicals and pharmaceuticals,” said Zhenxing Feng, associate professor of chemical engineering. “However, for the reaction to be economically viable, a catalyst such as palladium or platinum is invariably required to increase its reaction rate and thus lower cost.”
A modified manufacturing process for electric vehicle batteries, developed by University of Michigan engineers, could enable high ranges and fast charging in cold weather, solving problems that are turning potential EV buyers away.
“We envision this approach as something that EV battery manufacturers could adopt without major changes to existing factories,” said Neil Dasgupta, U-M associate professor of mechanical engineering and materials science and engineering, and corresponding author of the study published in Joule.
“For the first time, we’ve shown a pathway to simultaneously achieve extreme fast charging at low temperatures, without sacrificing the energy density of the lithium-ion battery.”