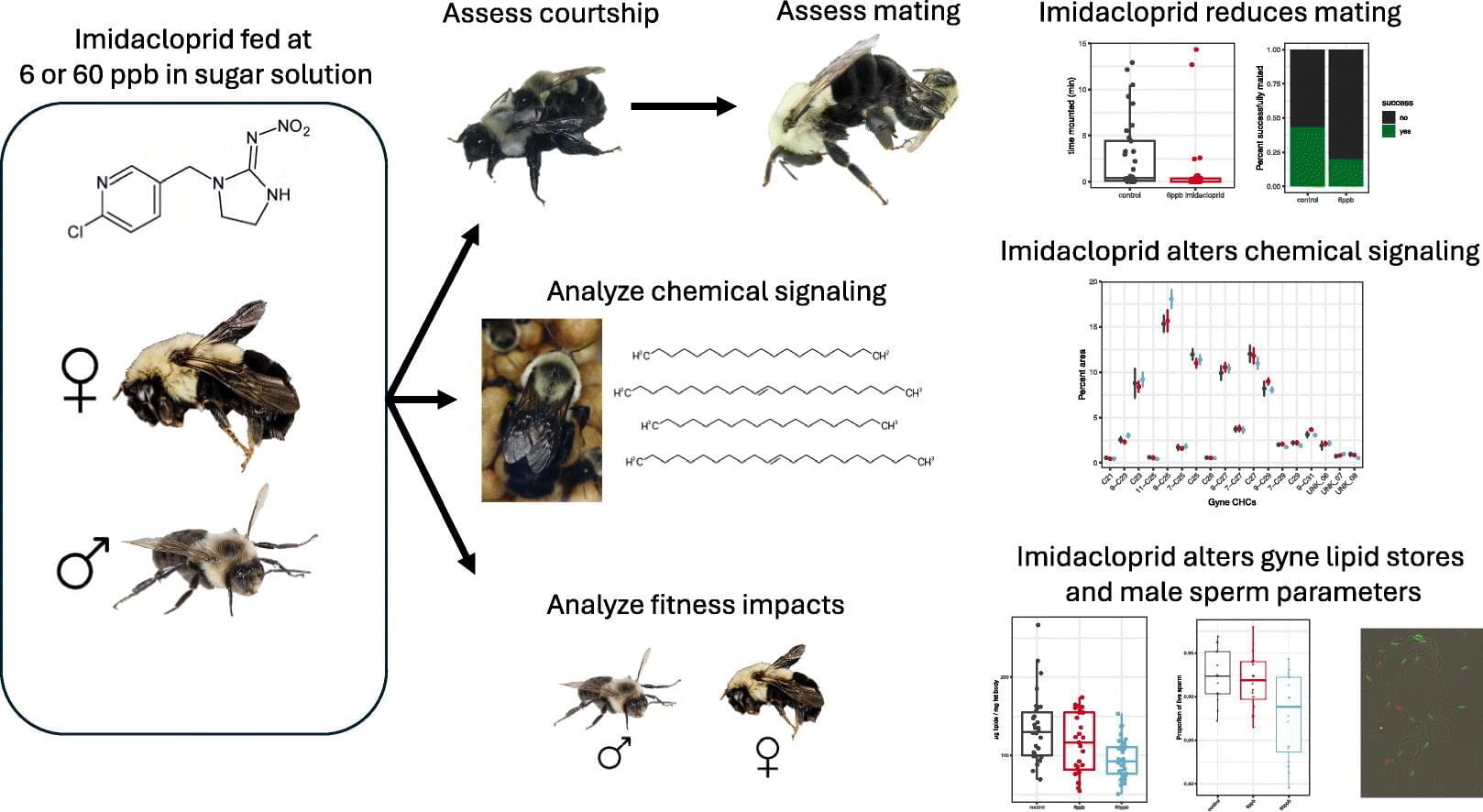
Insecticides can help protect crops against troublesome pests, but they also pose a risk for beneficial insects such as pollinators. A study led by researchers at Penn State provides insight into how even sublethal doses of insecticides can negatively affect pollinators by disrupting the mating process.
The study, published in the journal Science of The Total Environment, looked at the effects of imidacloprid, a neonicotinoid that is among the most widely used insecticides globally.
The researchers found that exposure to the insecticide, even at sublethal levels, reduced successful mating in bumble bees and altered the chemical signaling of both males and gynes—female bees capable of reproduction. It also negatively impacted both sperm viability in males and lipid storage in gynes.