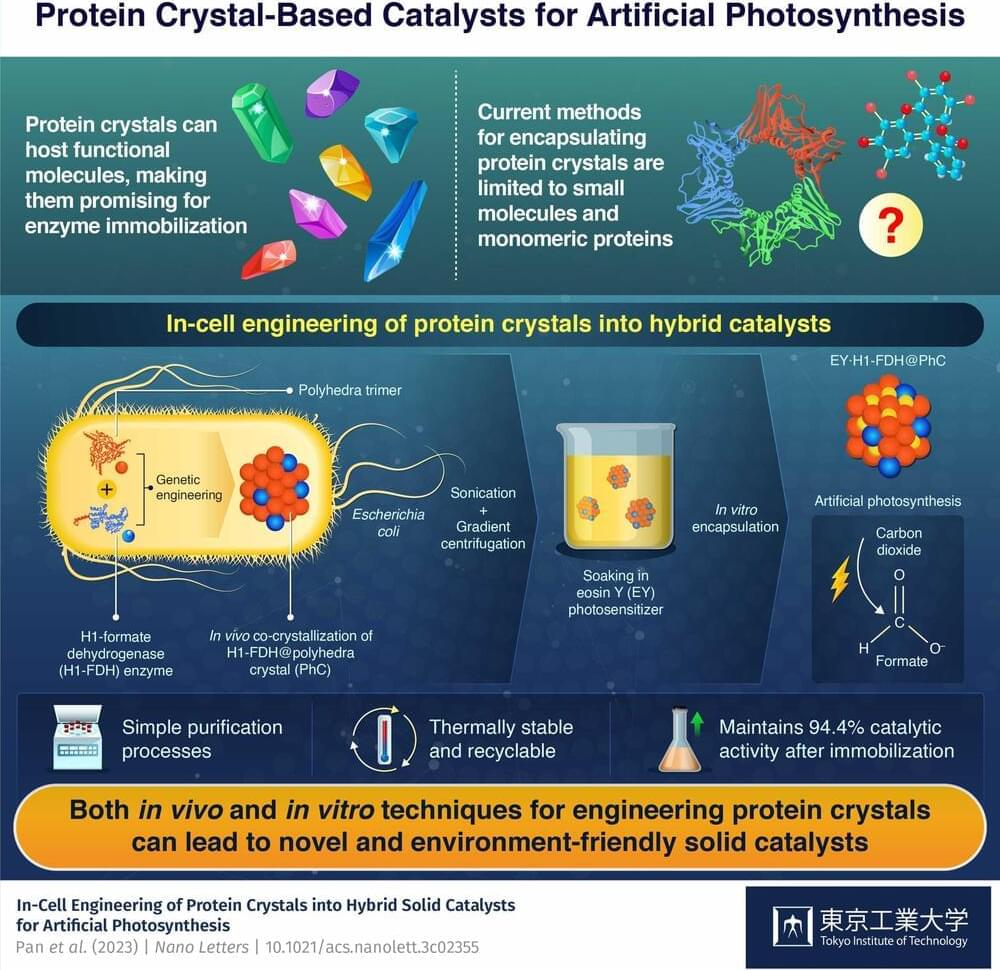
Recent developments in various domains have led to a growing interest in the potential of artificial intelligence to enhance our lives and environments. In particular, the application of artificial intelligence in the management of complex human diseases, such as cancer, has garnered significant attention. The evolution of artificial intelligence is thought to be influenced by multiple factors, including human intervention and environmental factors. Similarly, tumors, being heterogeneous and complex diseases, continue to evolve due to changes in the physical, chemical, and biological environment. Additionally, the concept of cellular intelligence within biological systems has been recognized as a potential attribute of biological entities. Therefore, it is plausible that the tumor intelligence present in cancer cells of affected individuals could undergo super-evolution due to changes in the pro-tumor environment. Thus, a comparative analysis of the evolution of artificial intelligence and super-complex tumor intelligence could yield valuable insights to develop better artificial intelligence-based tools for cancer management.
Tumor evolution refers to the changes that occur in a cancerous tumor over time as it grows and spreads (Hanahan and Weinberg, 2011; Lyssiotis and Kimmelman, 2017). These changes are the result of genetic mutations and changes in gene expression that can give rise to new subpopulations of cells within the tumor (Lyssiotis and Kimmelman, 2017; Balaparya and De, 2018). Over time, these subpopulations may accumulate subsequent mutations that confer enhanced survival and heightened proliferative capacity, thereby culminating in the emergence of a more formidable tumor exhibiting either heightened aggressiveness or treatment resistance (Balaparya and De, 2018; Gui and Bivona, 2022; Shin and Cho, 2023). Tumor evolution can have important implications for cancer diagnosis and treatment.