Apple could once again be hoping to lock its competitors out of the market, at least for a little while.
Category: futurism – Page 118
An exploration of the various Dyson sphere and swarm concepts and their practicality. My Patreon Page: / johnmichaelgodier My Event Horizon Channel:
/ eventhorizonshow Music: Cylinder Eight by Chris Zabriskie is licensed under a Creative Commons Attribution 4.0 license. https://creativecommons.org/licenses/.… https://chriszabriskie.com/cylinders/ Cylinder Five by Chris Zabriskie is licensed under a Creative Commons Attribution 4.0 license. https://creativecommons.org/licenses/.… https://chriszabriskie.com/cylinders/ Space Xplorers by Kevin MacLeod is licensed under a Creative Commons Attribution 4.0 license. https://creativecommons.org/licenses/.… http://incompetech.com/music/royalty–… Darkest Child by Kevin MacLeod is licensed under a Creative Commons Attribution 4.0 license. https://creativecommons.org/licenses/.… http://incompetech.com/music/royalty–… Intermission in D by Miguel Johnson https://migueljohnson.bandcamp.com/
【Qualia Structure Summer School】https://en.qualia-structure.jp/news/detail/3569We are planning to hold the sum…
Copilot+ PCs are the fastest, most intelligent, and longest lasting Windows PCs ever built. Subscribe to Microsoft on YouTube here: https://aka.ms/SubscribeT…
From Microsoft.
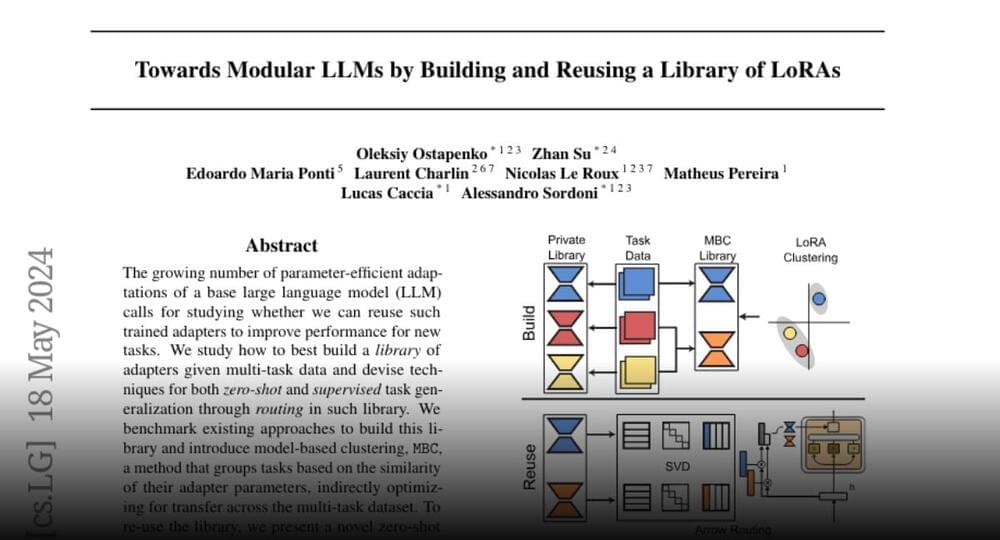
Towards Modular LLMs by Building and Reusing a Library of LoRAs https://huggingface.co/papers/2405.
The growing number of parameter-efficient adaptations of a base large language model (LLM) calls for studying whether we can reuse such trained adapters to improve performance for…
Join the discussion on this paper page.
The future of optical communications just got brighter. In a development reported in Advanced Photonics, researchers from Nanjing University have introduced iso-propagation vortices (IPVs), a novel concept that offers a solution to a long-standing challenge faced by scientists and engineers: how to increase information processing capacity while overcoming the limitations of traditional vortex beams.
Researchers used sound to reveal hidden patterns in protein folding, emphasizing the role of hydrogen bonds and water molecules in shaping protein structures.
Scientists have transformed their data into sounds to uncover how hydrogen bonds contribute to the lightning-fast gyrations that transform a string of amino acids to fold into a functional protein. Their study, published in the Proceedings of the National Academy of Sciences, offers an unprecedented view of the sequence of hydrogen-bonding events that occur when a protein morphs from an unfolded to a folded state.
“A protein must fold properly to become an enzyme or signaling molecule or whatever its function may be — all the many things that proteins do in our bodies,” said University of Illinois Urbana-Champaign chemistry professor Martin Gruebele, who led the new research with composer and software developer Carla Scaletti.
Testing two families of large language models (LLMs) (GPT and LLaMA2) on a battery of measurements spanning different theory of mind abilities, Strachan et al. find that the performance of LLMs can mirror that of humans on most of these tasks. The authors explored potential reasons for this.
Professor Barry Kemp, who has died the day after his 84th birthday, was an eminent Egyptologist who directed the excavations at the site in Middle Egypt known as Amarna.
‘The danger of being an absolute ruler is that no one dares tell you that what you have just decreed is not a good idea’