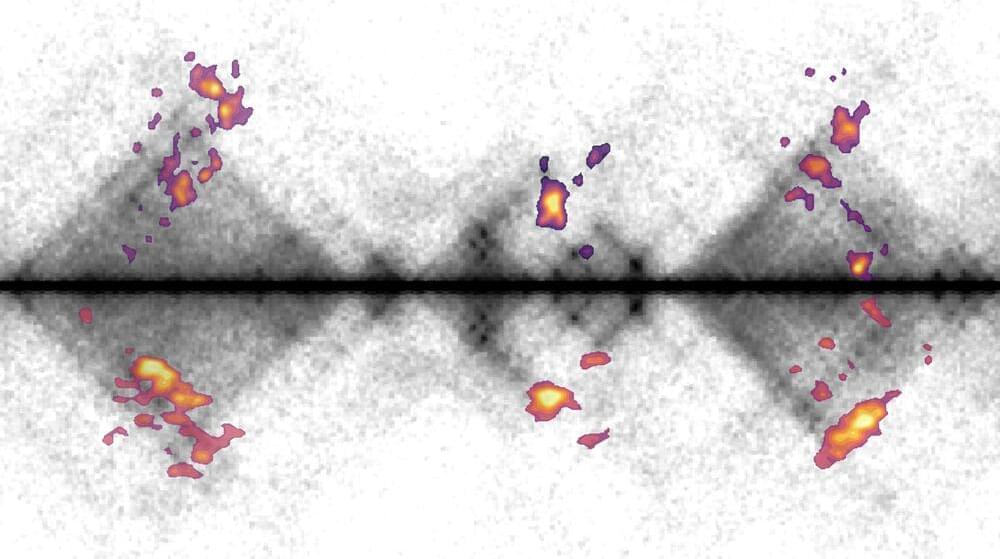
New research from the Kind Group at the Hubrecht Institute sheds light on how cells repair damaged DNA. For the first time, the team has mapped the activity of repair proteins in individual human cells.
The study demonstrates how these proteins collaborate in so-called “hubs” to repair DNA damage. This knowledge offers opportunities to improve cancer therapies and other treatments where DNA repair is essential. The researchers published their findings in Nature Communications on November 21.
DNA is the molecule that carries our genetic information. It can be damaged by normal cellular processes as well as external factors such as UV radiation and chemicals. Such damage can lead to breaks in the DNA strand. If DNA damage is not properly repaired, mutations can occur, which may result in diseases like cancer. Cells use repair systems to fix this damage, with specialized proteins locating and binding to the damaged regions.