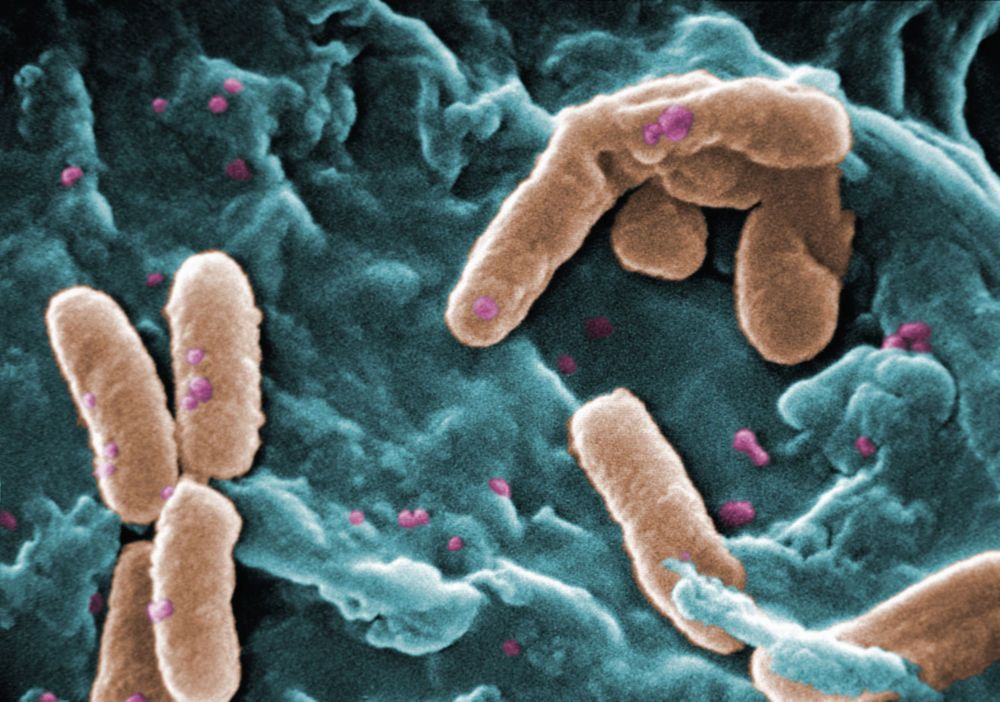
New genetic engineering method promises to super-charge recombineering and open the bacterial world at large to this underutilized approach.
Category: bioengineering – Page 126
AI And The Digital Mine
Posted in bioengineering, climatology, robotics/AI
When you think of the words “data” and “mine”, no doubt the idea of data mining comes first. However, just as much as we find value in mining the rich resources of data, so too can we apply the advanced techniques for dealing with data to real-world mining — that is, extracting natural resources from the earth. The world is just as dependent on natural resources as it is data resources, so it makes sense to see how the evolving areas of artificial intelligence and machine learning have an impact on the world of mining and natural resource extraction.
Mining has always been a dangerous profession, since extracting minerals, natural gas, petroleum, and other resources requires working in conditions that can be dangerous for human life. Increasingly, we are needing to go to harsher climates such as deep under the ocean or deep inside the earth to extract the resources we still need. It should come as little surprise then that mining and resource extraction companies are looking to robotics, autonomous systems, and AI applications of all sorts to minimize risk, maximize return, and also lessen the environmental impact that mining has on our ecosystem.
On a recent AI Today podcast episode, Antoine Desmet of mining technology and equipment company Komatsu shared how they’re using advanced forms of AI, automation, and robotics to make an impact on the organization’s operations. Antoine has an interesting background, starting his career as a telecom engineer and receiving a Ph.D in neural network engineering. After getting his Ph.D, he returned to Komatsu and started working in surface analytics. He states that at the time there was a lot of data to work with, but very few analytics in place. He decided to start implementing machine learning and in the last few years his company has seen significant growth through this approach, with his data science team growing from just one person to ten people.
Not all viruses set out to cause widespread death and sickness — some have the potential to fight cancer, according to new research.
Researchers from Hokkaido University in Japan have genetically engineered adenoviruses, which is a family of viruses that cause mild symptoms, to replicate inside cancer cells and kill them, according to a new paper in the journal Cancers.
To do this, Fumihiro Higashino, a molecular oncologist, and his team inserted adenylate-uridylate-rich elements (AREs) from two human genes — a stabilizing element found in a type of macromolecule present in all biological cells — into two strains of the virus to help specifically attack cancer cells.
Circa 2019 face_with_colon_three
Multipotent cells are critical to regenerative medicine and its associated deployment strategies. Localizing an abundant source of autologous, adult stem cells circumvents the immunological prohibitions of allogeneity and ethical dilemmas of embryologic stem cells, respectively. Classically, these cells have been described as mesenchymal stem cells (MSCs). In this chapter, we characterize adipose tissue as a unique source of MSCs because of its ubiquity, redundancy, and procurability. Specifically, lipoaspirates can be minimally processed to provide a heterogenous, cell-dense isolate – the stromal vascular fraction (SVF) – composed of terminally differentiated vessel-associated cell lines as well as putative progenitor cells. These cells have been cultured and expanded, giving rise to a dynamic cell line termed adipose-derived stromal cells (ASCs). SVF and ASC cell isolates are often administered by standard clinical routes including parenteral, topical application, and local injection in the clinical translational studies of cardiovascular ischemia, neurological injury, rheumatologic and orthopedic disease as well as advanced wound care and tissue engineering. These clinical applications raise safety concerns specific to administration, sequestration, and tumor growth augmentation. Further studies SVF and ASC cells are necessary to realize their potential in a regenerative medicine capacity.
I always enjoy the perspective of David Wood, and in this session of the London Futurists there is a panel discussion about genetic engineering in the future.
Our DNA is becoming as readable, writable, and hackable as our information technology. The resulting genetic revolution is poised to transform our healthcare, our choices for the characteristics of the next generation, and our evolution as a species. The future could bring breathtaking advances in human well-being, but it could also descend into a dangerous genetic arms race.
These claims are made in the recent book “Hacking Darwin: Genetic Engineering and the Future of Humanity”, https://hackingdarwin.com/ by Technology Futurist Jamie Metzl, https://jamiemetzl.com/
Jamie’s view is that society isn’t at all ready for the fast-approaching future of widespread genetic hacking.
Here is some feedback for his book:
After biomedical scientists demonstrated that they could make dangerous viruses like influenza even more dangerous, the National Institutes of Health (NIH) implemented a three-year moratorium on funding such research. But a couple of months ago, in December, the moratorium was lifted, and a tight set of rules were put in its place, such as a mandate for oversight panels.
The prospect of engineering a deadly pandemic virus in a laboratory suggests that only a fool would wish away government regulation entirely.
However, as a whole, regulation has done more harm than good in the arena of scientific innovation. The reason is that the sort of person who thinks like a bureaucratic regulator isn’t the sort of person who thinks like a scientist. The sad fact of the matter is that those most interested in the regulatory process tend to be motivated by politics and ideology rather than scientific inquiry and technological progress.
INDIANAPOLIS (WISH) — First is was monkeys, then dogs.
Now, researchers are turning to cows in hopes of developing a treatment for the coronavirus.
Scientists at SAb Biotherapeutics in South Dakota created an embryo via genetic engineering that contains human chromosomes. The embryo was then implanted into cattle. The cows gave birth to calves that internally function similarly to a person, specifically with regards to the human immune system.
Then there is the COVID-19 Open Research Dataset (CORD-19), a multi-institutional initiative that includes The White House Office of Science and Technology Policy, Allen Institute for AI, Chan Zuckerberg Initiative (CZI), Georgetown University’s Center for Security and Emerging Technology (CSET), Microsoft, and the National Library of Medicine (NLM) at the National Institutes of Health (NIH).
The goal of this initiative is to create new natural language processing and machine learning algorithms to scour scientific and medical literature to help researchers prioritize potential therapies to evaluate for further study. AI is also being used to automate screening at checkpoints by evaluating temperature via thermal cameras, as well as modulations in sweat and skin discoloration. What’s more, AI-powered robots have even been used to monitor and treat patients. In Wuhan, the original epicenter of the pandemic, an entire field hospital was transitioned into a “smart hospital” fully staffed by AI robotics.
Any time of great challenge is a time of great change. The waves of technological innovation that are occurring now will echo throughout eternity. Science, technology, engineering and mathematics are experiencing a call to mobilization that will forever alter the fabric of discovery in the fields of bioengineering, biomimicry and artificial intelligence. The promise of tomorrow will be perpetuated by the pangs of today. It is the symbiosis of all these fields that will power future innovations.