Jan 22, 2023
Cellular Reprogramming Extends Lifespan in Mice, Longevity Startup Says
Posted by Dan Breeden in categories: biotech/medical, genetics, life extension
Cellular reprogramming builds on the Nobel Prize-winning work of Shinya Yamanaka, who showed that adult cells could be transformed back into stem cells by exposing them to a specific set of genome-regulating proteins known as transcription factors. The Salk team’s innovation was to reduce the exposure times to the so-called Yamanaka factors, which they found could reverse epigenetic changes to the cells without reverting them to stem cells.
While the approach led to clear increases in lifespan in prematurely aging mice, the fact that no one had been able to replicate the result in healthy mice since then raised doubts about the approach. “Different groups have tried this experiment, and the data have not been positive so far,” Alejandro Ocampo, from the University of Lausanne in Switzerland, who carried out the original Salk experiments, told MIT Technology Review.
But now, Rejuvenate Bio claims that when they exposed healthy mice near the end of their lives to a subset of the Yamanaka factors, they lived for another 18 weeks on average, compared to just 9 weeks for those that didn’t undergo cellular reprogramming.

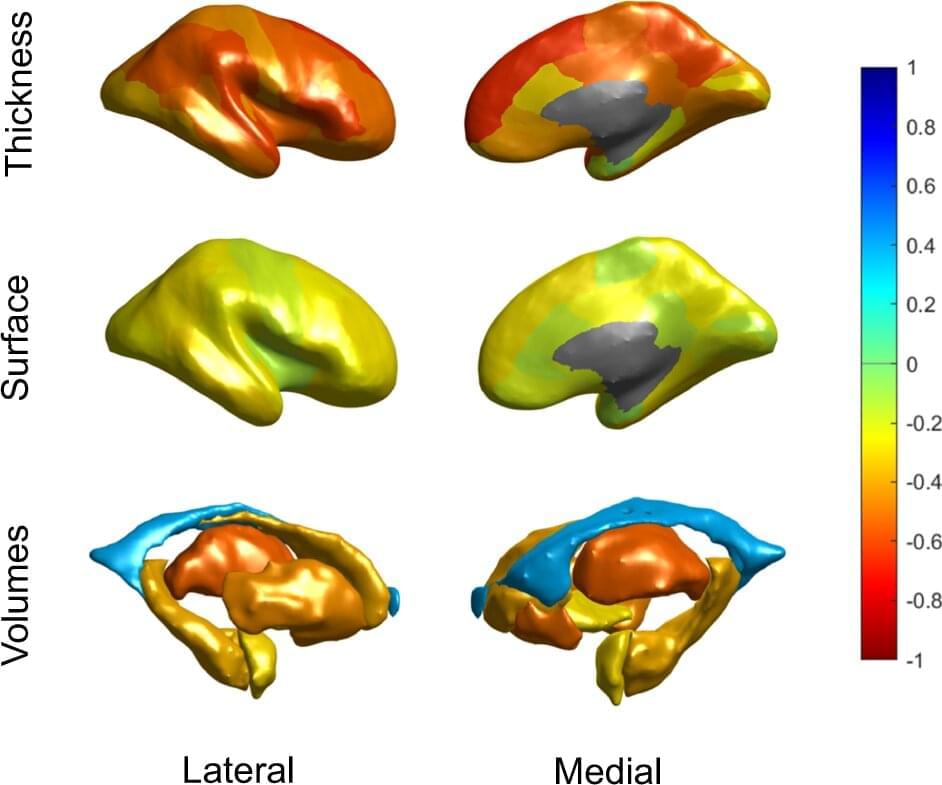
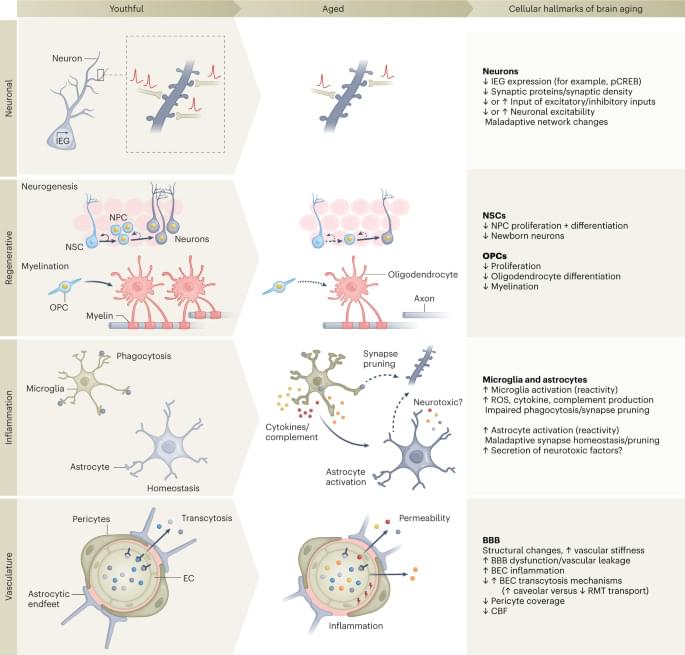
 Aging is a complex process best characterized as the chronic dysregulation of cellular processes leading to deteriorated tissue and organ function. While aging cannot currently be prevented, its impact on lifespan and healthspan in the elderly can potentially be minimized by interventions that aim to return these cellular processes to optimal function. Recent studies have demonstrated that partial reprogramming using the Yamanaka factors (or a subset; OCT4, SOX2, and KLF4; OSK) can reverse age-related changes in vitro and in vivo. However, it is still unknown whether the Yamanaka factors (or a subset) are capable of extending the lifespan of aged wild type mice. Here, we show that systemically delivered AAVs, encoding an inducible OSK system, in 124-week-old mice extends the median remaining lifespan by 109% over wild-type controls and enhances several health parameters. Importantly, we observed a significant improvement in frailty scores indicating that we were able to improve the healthspan along with increasing the lifespan. Furthermore, in human keratinocytes expressing exogenous OSK, we observed significant epigenetic markers of age-reversal, suggesting a potential reregulation of genetic networks to a younger, potentially healthier state. Together, these results may have important implications for the development of partial reprogramming interventions to reverse age-associated diseases in the elderly.
Aging is a complex process best characterized as the chronic dysregulation of cellular processes leading to deteriorated tissue and organ function. While aging cannot currently be prevented, its impact on lifespan and healthspan in the elderly can potentially be minimized by interventions that aim to return these cellular processes to optimal function. Recent studies have demonstrated that partial reprogramming using the Yamanaka factors (or a subset; OCT4, SOX2, and KLF4; OSK) can reverse age-related changes in vitro and in vivo. However, it is still unknown whether the Yamanaka factors (or a subset) are capable of extending the lifespan of aged wild type mice. Here, we show that systemically delivered AAVs, encoding an inducible OSK system, in 124-week-old mice extends the median remaining lifespan by 109% over wild-type controls and enhances several health parameters. Importantly, we observed a significant improvement in frailty scores indicating that we were able to improve the healthspan along with increasing the lifespan. Furthermore, in human keratinocytes expressing exogenous OSK, we observed significant epigenetic markers of age-reversal, suggesting a potential reregulation of genetic networks to a younger, potentially healthier state. Together, these results may have important implications for the development of partial reprogramming interventions to reverse age-associated diseases in the elderly.