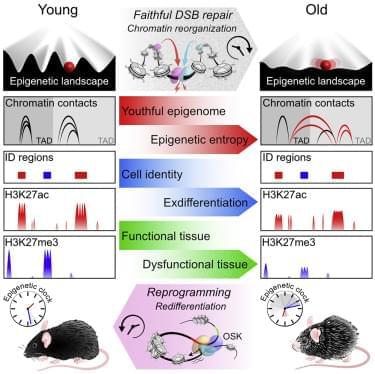
The rebooting came in the form of a gene therapy involving three genes that instruct cells to reprogram themselves—in the case of the mice, the instructions guided the cells to restart the epigenetic changes that defined their identity as, for example, kidney and skin cells, two cell types that are prone to the effects of aging. These genes came from the suite of so-called Yamanaka stem cells factors—a set of four genes that Nobel scientist Shinya Yamanaka in 2006 discovered can turn back the clock on adult cells to their embryonic, stem cell state so they can start their development, or differentiation process, all over again. Sinclair didn’t want to completely erase the cells’ epigenetic history, just reboot it enough to reset the epigenetic instructions. Using three of the four factors turned back the clock about 57%, enough to make the mice youthful again.
“We’re not making stem cells, but turning back the clock so they can regain their identity,” says Sinclair. “I’ve been really surprised by how universally it works. We haven’t found a cell type yet that we can’t age forward and backward.”
Rejuvenating cells in mice is one thing, but will the process work in humans? That’s Sinclair’s next step, and his team is already testing the system in non-human primates. The researchers are attaching a biological switch that would allow them to turn the clock on and off by tying the activation of the reprogramming genes to an antibiotic, doxycycline. Giving the animals doxycycline would start reversing the clock, and stopping the drug would halt the process. Sinclair is currently lab-testing the system with human neurons, skin, and fibroblast cells, which contribute to connective tissue.