Jul 23, 2024
Whoever Controls Electrolytes will Pave the way for Electric Vehicles
Posted by Dan Breeden in categories: chemistry, energy, sustainability, transportation
Whoever Controls #Electrolytes will Pave the way for #ElectricVehicles.
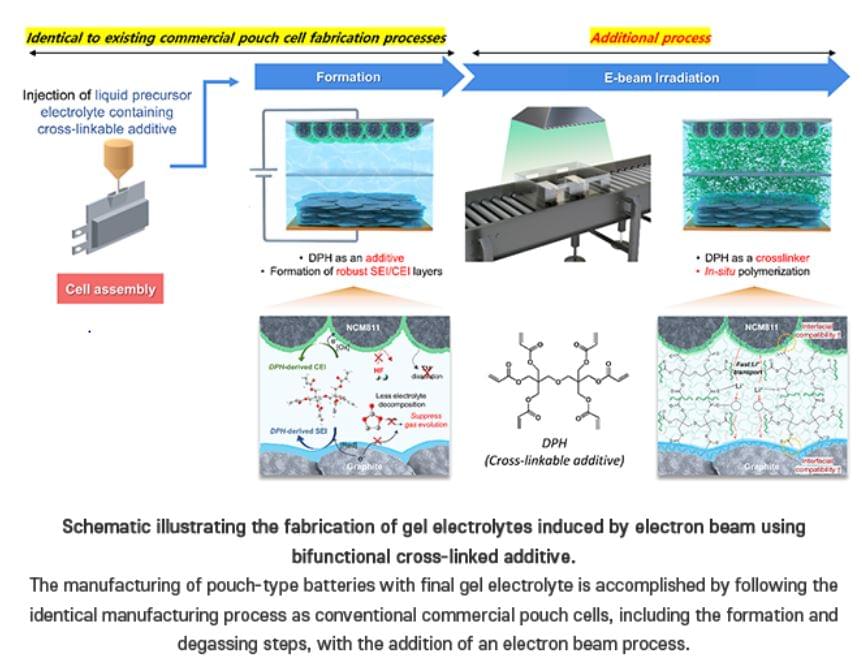
Team from the Dept of Chemistry at POSTECH have achieved a breakthrough in creating a gel electrolyte-based battery that is both stable and commercially viable…
Team develops a commercially viable and safe gel electrolyte for lithium batteries. Professor Soojin Park, Seoha Nam, a PhD candidate, and Dr. Hye Bin Son from the Department of Chemistry at Pohang University of Science and Technology (POSTECH) have achieved a breakthrough in creating a gel electrolyte-based battery that is both stable and commercially viable. Their research was recently published in the international journal Small.
Continue reading “Whoever Controls Electrolytes will Pave the way for Electric Vehicles” »