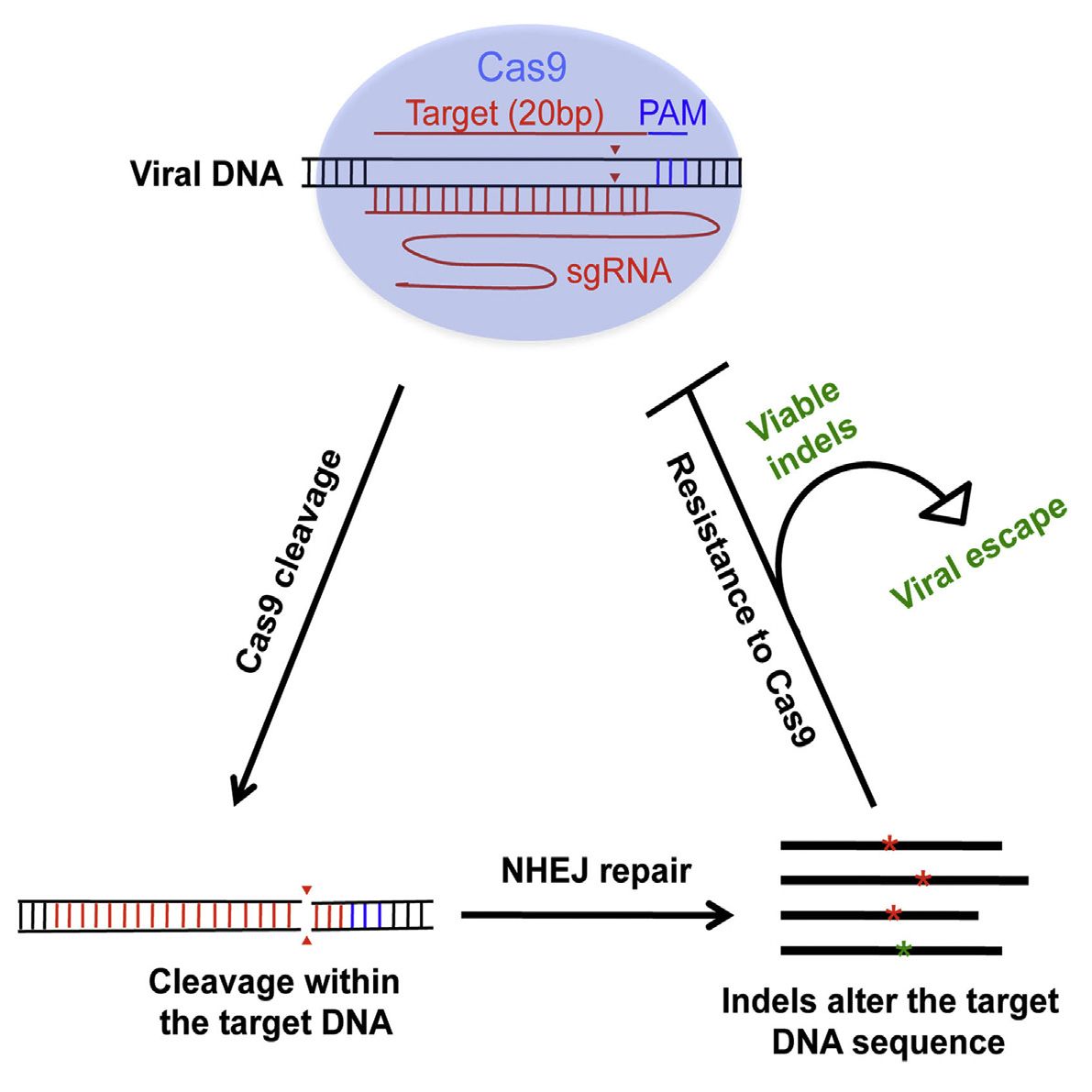
The CRISPR/Cas9 gene-editing platform may need a little bit more tweaking before it can be used as an effective antiviral, reports a study published April 7 in Cell Reports. Researchers who used CRISPR/Cas9 to mutate HIV-1 within cellular DNA found that while single mutations can inhibit viral replication, some also led to unexpected resistance. The researchers believe targeting multiple viral DNA regions may be necessary for the potential antiviral aspect of CRISPR/Cas9 to be effective.
Upon entry into a cell, HIV’s RNA genome is converted into DNA and becomes entwined with the cellular DNA. From here, CRISPR/Cas9 can be programmed to target a DNA sequence and cleave viral DNA. The problem is that HIV is notoriously good at surviving and thriving with new mutations, so while many viruses are killed by the targeted approach, those that escape the CRISPR/Cas9 treatment become more difficult to target.
“When we sequence the viral RNA of escaped HIV, the surprise is that the majority of the mutations that the virus has are nicely aligned at the site where Cas9 cleaves the DNA, which immediately indicates that these mutations, instead of resulting from the errors of viral reverse transcriptase, are rather introduced by the cellular non-homologous end joining machinery when repairing the broken DNA,” says senior study author Chen Liang, Senior Investigator at the Lady Davis Institute at the Jewish General Hospital and the Associate Professor of Medicine at the McGill University AIDS Centre.








Comments are closed.