Archive for the ‘satellites’ category: Page 162
Aug 7, 2018
SpaceX Falcon 9 Lifts Off In Florida, Places Indonesian Satellite In Orbit
Posted by Genevieve Klien in category: satellites
The launch marks the first reuse of an improved Falcon 9 “Block 5,” which includes several upgrades designed to allow SpaceX to quickly refurbish and re-launch the rocket.
Aug 7, 2018
SpaceX’s brand new, recyclable Falcon 9 rocket launches again
Posted by Genevieve Klien in category: satellites

In the tiny hours of Tuesday morning, SpaceX launched an Indonesian satellite in its 15th flight this year.
It’s also the first re-flight of the company’s new, recyclable Falcon 9 Block 5 rocket, which had its first launch back in May.
Continue reading “SpaceX’s brand new, recyclable Falcon 9 rocket launches again” »
Aug 2, 2018
Made In Space’s ‘Archinaut’ Could Build Big Power Systems for Small Satellites
Posted by Klaus Baldauf in categories: robotics/AI, satellites, solar power, sustainability
Small satellites will soon pack an outsize power punch, if one company’s plans come to fruition.
One of the first big jobs for the Archinaut in-space assembly robot being developed by California startup Made In Space may involve outfitting small satellites with large solar-power systems in Earth orbit.
Such work could boost the power potential of spacecraft in the 330-lb. to 660-lb. (150 to 300 kilograms) range by a factor of five or more, allowing them to take on duties previously limited to larger satellites, company representatives said. [Satellite Quiz: How Well Do You Know What’s Orbiting Earth?].
Continue reading “Made In Space’s ‘Archinaut’ Could Build Big Power Systems for Small Satellites” »
Jul 27, 2018
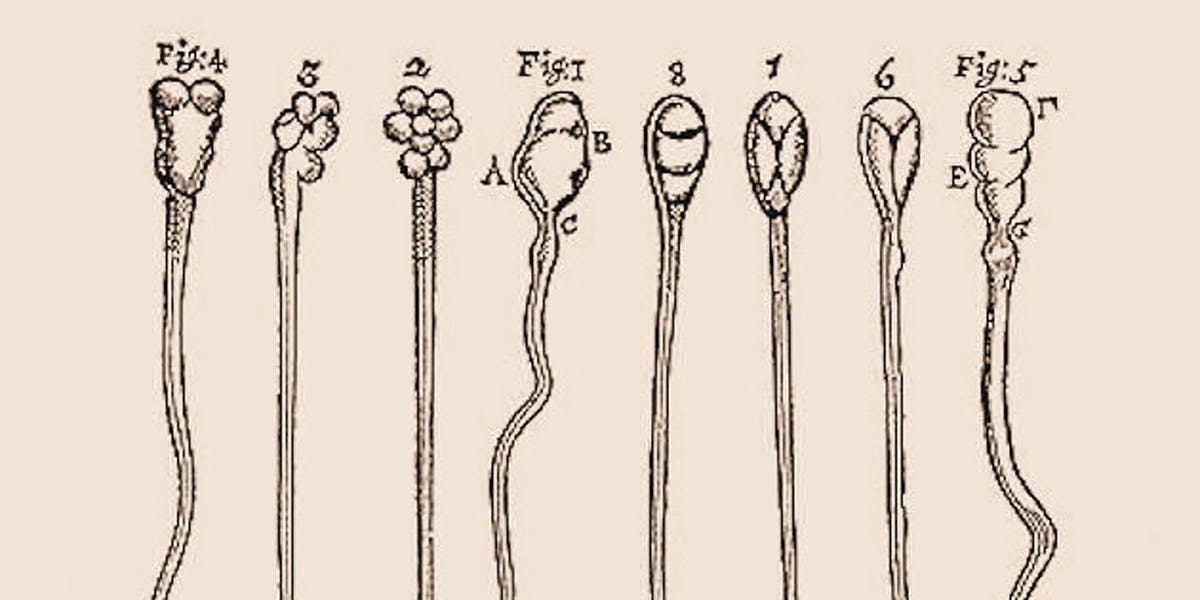
Changes in Sperm RNA May Shape Future Generations, Scientists Warn
Posted by Genevieve Klien in categories: biotech/medical, ethics, satellites
While the rest of the world debates the ethics of designer babies, a team at the University of Massachusetts Medical School (UMass) have shown that we might not need CRISPR to change the genes of future generations. Their paper, released this week in the journal Developmental Cell, shows that things like diet and stress might affect some crucial genetic components of sperm, and that these tiny changes have real effects on how babies develop.
The same way rockets bound for outer space contain “payloads” like satellites, or astronauts who battle giant balls of urine, sperm are also like little rockets containing their own cargo: “small RNAs.” This study found that not only do RNA sequences play a crucial role in how genes get expressed early on in human development, but they can also be radically changed by the lifestyles of fathers. Things like diet, and in particular, stress can change the makeup of this crucial RNA cargo and lead to observable changes in offspring, says researcher Colin Conine, Ph.D., at UMass Medical School’s Rando Lab.
“Labs all over the world have been able to link changes in dad’s lifestyle to changes in RNA in the sperm, and then that leads to phenotypes in the offspring,” Conine tells Inverse. “Our study was one of the first to really look at how changes small RNAs affect early development. We wanted to ask, what are the first steps that lead to these phenotypes down the road?”
Jul 24, 2018
SpaceX launches record-setting satellite with a block 5 Falcon 9
Posted by Genevieve Klien in categories: Elon Musk, satellites
Elon Musk’s rocket company launched the final iteration of its workhorse rocket carrying the biggest communications satellite ever.
Jul 13, 2018
Satellite startups turn to reinventing broadband, mapping and other industries
Posted by Klaus Baldauf in categories: computing, mapping, mobile phones, satellites
Smartphones have disrupted transportation, payments and communication. But the underlying technology has tangentially changed a completely different sector: satellites.
The advances made in miniaturizing technologies that put a computer in your pocket — cameras, batteries, processors, radio antennas — have also made it easier and cheaper for entrepreneurs to launch matter into space. And investors are taking notice.
The chart below shows worldwide venture and PE investment in satellite technology companies.
Continue reading “Satellite startups turn to reinventing broadband, mapping and other industries” »
Jul 13, 2018
Giant Satellite Fuel Tank Sets New Record for 3D Printed Space Parts
Posted by Klaus Baldauf in categories: 3D printing, energy, satellites
DENVER, July 11, 2018 /PRNewswire/ — Lockheed Martin (NYSE: LMT) has embraced a 3D printed titanium dome for satellite fuel tanks so big you can’t even put your arms around it. The 46-inch- (1.16-meter-) diameter vessel completed final rounds of quality testing this month, ending a multi-year development program to create giant, high-pressure tanks that carry fuel on board satellites.

The titanium tank consists of three parts welded together: two 3D printed domes that serve as caps, plus a variable-length, traditionally-manufactured titanium cylinder that forms the body.
Continue reading “Giant Satellite Fuel Tank Sets New Record for 3D Printed Space Parts” »
Congrats!
Pinaplano na ng DOST ang paggawa ng cube satellites dito sa Pilipinas matapos makarating sa outer space ang unang cube satellite na gawang Pinoy.