Archive for the ‘particle physics’ category: Page 90
May 1, 2024
‘QBism’: The most radical interpretation of quantum mechanics ever
Posted by Dan Breeden in categories: mathematics, particle physics, quantum physics
Quantum mechanics, the most potent theory physicists have developed, doesn’t make sense. What I mean by that statement is that quantum mechanics — which was developed to describe the microworld of molecules, atoms, and subatomic particles — leaves its users without a common-sense picture of what it describes. Full of what seem to be paradoxes and puzzles, quantum physics demands, for most scientists, an interpretation: a way of making sense of its mathematical formalism in terms of a concrete description of what exists in the world and how we interact with it. Unfortunately, after a century not one but a basketful of “quantum interpretations” have been proposed. Which one is correct? Which one most clearly understands what quantum physics has been trying to tell us these past 100 years?
In light of these questions, I’m beginning a series that explores the most radical of all the quantum interpretations, the one I think gets it right, or at least is pointed in the right direction. It is a relative newcomer to the scene, so you may not have heard of it. But it has been gaining a lot of attention recently because it doesn’t just ask us to reimagine how we view the science of atoms; it asks us to reimagine the process of science itself.
The term “QBism” was shorthand for “Quantum Bayesianism” when this idea/theory/interpretation was first proposed in the late 1990s and early 2000s. The name hit the nail on the head because “Bayesianism” is a radical way of interpreting probabilities. The Bayesianist approach to what we mean by probability differs strongly from what you learned in school about coin flips and dice rolls and how frequently a particular result can be expected to appear. Since probabilities lie at the heart of quantum mechanics, QBism zeroed in on a key aspect of quantum formalism — one that other interpretations had missed or swept under the rug — because it focused squarely on how we interpret probabilities. We’re going to dig deep into all of this as we go along in this series, but since today’s column is supposed to be the introduction, let’s start with a 10,000-foot view of what’s at stake in the great “Quantum Interpretation Wars” so we can see where QBism fits in.
May 1, 2024
The science of static shock jolted into the 21st century
Posted by Shailesh Prasad in categories: bioengineering, biological, chemistry, computing, mathematics, particle physics, science
Now Princeton researchers have sparked new life into static. Using millions of hours of computational time to run detailed simulations, the researchers found a way to describe static charge atom-by-atom with the mathematics of heat and work. Their paper appeared in Nature Communications on March 23.
The study looked specifically at how charge moves between materials that do not allow the free flow of electrons, called insulating materials, such as vinyl and acrylic. The researchers said there is no established view on what mechanisms drive these jolts, despite the ubiquity of static: the crackle and pop of clothes pulled from a dryer, packing peanuts that cling to a box.
“We know it’s not electrons,” said Mike Webb, assistant professor of chemical and biological engineering, who led the study. “What is it?”
May 1, 2024
Machine learning and theory
Posted by Dan Kummer in categories: information science, mathematics, particle physics, quantum physics, robotics/AI
Theoretical physicists employ their imaginations and their deep understanding of mathematics to decipher the underlying laws of the universe that govern particles, forces and everything in between. More and more often, theorists are doing that work with the help of machine learning.
As might be expected, the group of theorists using machine learning includes people classified as “computational” theorists. But it also includes “formal” theorists, the people interested in the self-consistency of theoretical frameworks, like string theory or quantum gravity. And it includes “phenomenologists,” the theorists who sit next to experimentalists, hypothesizing about new particles or interactions that could be tested by experiments; analyzing the data the experiments collect; and using results to construct new models and dream up how to test them experimentally.
In all areas of theory, machine-learning algorithms are speeding up processes, performing previously impossible calculations, and even causing theorists to rethink the way theoretical physics research is done.
Apr 30, 2024
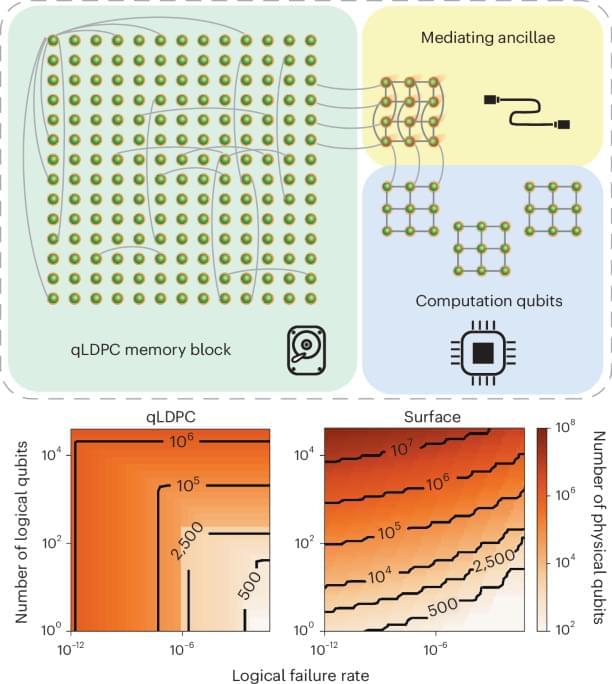
Constant-overhead fault-tolerant quantum computation with reconfigurable atom arrays
Posted by Dan Breeden in categories: particle physics, quantum physics
Quantum low-density parity-check codes are highly efficient in principle but challenging to implement in practice. This proposal shows that these codes could be implemented in the near term using recently demonstrated neutral-atom arrays.
Apr 30, 2024
Spintronics Breakthrough: Unlocking the Power of Radial Vortices
Posted by Dan Breeden in categories: materials, particle physics
A team at HZB has investigated a new, simple method at BESSY II that can be used to create stable radial magnetic vortices in magnetic thin films.
In some materials, spins form complex magnetic structures within the nanometre and micrometer scale in which the magnetization direction twists and curls along specific directions. Examples of such structures are magnetic bubbles, skyrmions, and magnetic vortices.
Spintronics aims to make use of such tiny magnetic structures to store data or perform logic operations with very low power consumption, compared to today’s dominant microelectronic components. However, the generation and stabilization of most of these magnetic textures is restricted to a few materials and achievable under very specific conditions (temperature, magnetic field…).
Apr 30, 2024
Do Magnetic Monopoles Exist?
Posted by Dan Breeden in categories: information science, particle physics, quantum physics
The elegant equations of classical electromagnetism written by James Clark Maxwell in 1861 display a remarkable symmetry between electric and magnetic fields except for their sources. We know about electric charges but we have not found magnetic charges. Bar magnets are dipoles with two poles, north and south, for the magnetic field, resembling the configuration of an electric field sourced by a pair of positive and negative electric charges. However, we had never seen experimental evidence for a magnetic monopole, namely a magnetic charge with only one magnetic pole, a net north or south, from where magnetic field lines emanate, just like the electric field sourced by an electric charge. In a symmetric theory of electromagnetism, magnetic monopoles should exist.
The existence of monopoles with a net magnetic charge was proposed by Paul Dirac in 1931 to explain the quantized (discrete) values of electric charges. Dirac found that magnetic charges should be an integer multiple of a fundamental unit, g_D, equal to the electron charge, e, divided by twice the fine-structure constant, or about 68.5e.
In classical physics, the existence of magnetic monopoles restores symmetry to Maxwell’s equations. But in the broader context of quantum mechanics, Gerard ‘t Hooft and Alexander Polyakov showed in 1974 that magnetic monopoles are required in Grand Unified Theories of the strong, weak and electromagnetic interactions. Since the electric charge is quantized, magnetic charges are unavoidable in these theories. Magnetic charges with the lowest mass must be stable because magnetic charge is conserved and they cannot decay into lower-mass particles.
Apr 29, 2024
Can Particles be Quantum Entangled Across Time?
Posted by Dan Breeden in categories: particle physics, quantum physics

1,769 views • Premiered 82 minutes ago • #worldsciencefestival #quantumentanglement #briangreene
Apr 29, 2024
Scientists Have Created a Functional Brain Cell Based on a Mix of Salt And Water
Posted by Paul Battista in categories: biological, neuroscience, particle physics
For the first time, researchers have simulated neurological junctions called synapses using the same water and salt ingredients the brain uses, contributing to an emerging field that combines biology with electronics called iontronics.
The team from Utrecht University in the Netherlands and Sogang University in South Korea claim to have been inspired by the functioning of the human brain, which also uses charged particles called ions dissolved in water to transmit signals within neurons.
An important feature of the brain’s ability to process information is synaptic plasticity, which allows neurons to adjust the strength of connections between them in response to input history.
Apr 29, 2024
Quantum Breakthrough when Light makes Materials Magnetic
Posted by Natalie Chan in categories: computing, particle physics, quantum physics
The potential of quantum technology is huge but is today largely limited to the extremely cold environments of laboratories. Now, researchers at Stockholm University, at the Nordic Institute for Theoretical Physics and at the Ca’ Foscari University of Venice have succeeded in demonstrating for the very first time how laser light can induce quantum behavior at room temperature — and make non-magnetic materials magnetic. The breakthrough is expected to pave the way for faster and more energy-efficient computers, information transfer and data storage.
Within a few decades, the advancement of quantum technology is expected to revolutionize several of society’s most important areas and pave the way for completely new technological possibilities in communication and energy.
Of particular interest for researchers in the field are the peculiar and bizarre properties of quantum particles — which deviate completely from the laws of classical physics and can make materials magnetic or superconducting.