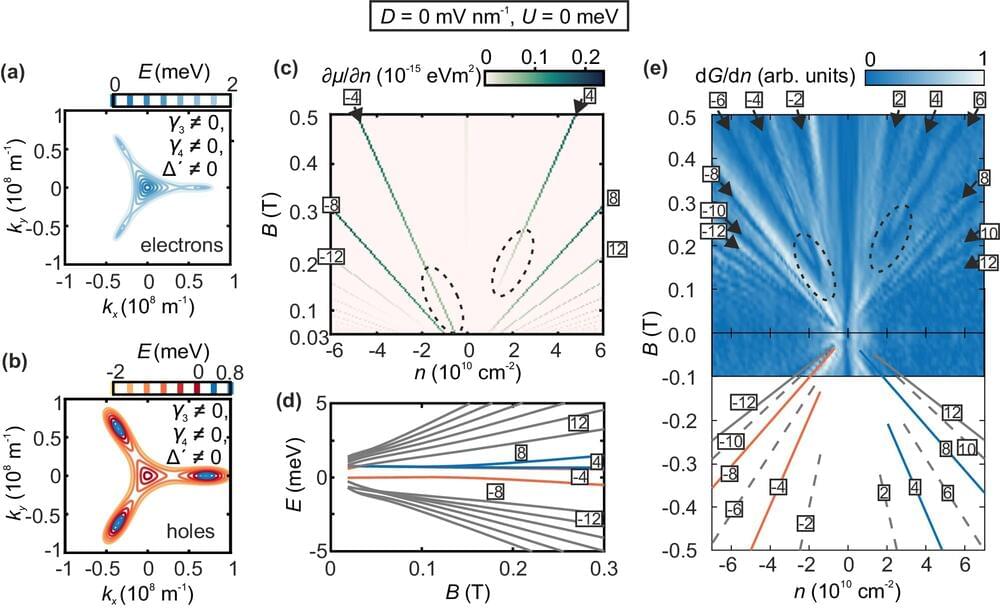
Photonic quantum computation, a type of quantum computation that uses light particles or photons, is divided into two main categories: discrete-variable (DV) and continuous-variable (CV) photonic quantum computation. Both have been realized experimentally and can be combined to overcome individual limitations. Photonic quantum computation is important as it can perform specific computational tasks more efficiently. It has several advantages, including the ability to observe and engineer quantum phenomena at room temperature, maintain coherence, and be engineered using mature technologies. The future of photonic quantum computing looks promising due to the significant progress in photonic technology.
Photonic quantum computation is a type of quantum computation that uses photons, particles of light, as the physical system for performing the computation. Photons are ideal for quantum systems because they operate at room temperature and photonic technologies are relatively mature. The field of photonic quantum computation is divided into two main categories: discrete-variable (DV) and continuous-variable (CV) photonic quantum computation.
In DV photonic quantum computation, quantum information is represented by one or more modal properties, such as polarization, that take on distinct values from a finite set. Quantum information is processed via operations on these modal properties and eventually measured using single photon detectors. On the other hand, in CV photonic quantum computation, quantum information is represented by properties of the electromagnetic field that take on any value in an interval, such as position. The electromagnetic field is transformed via Gaussian and non-Gaussian operations and then detected via homodyne detection.