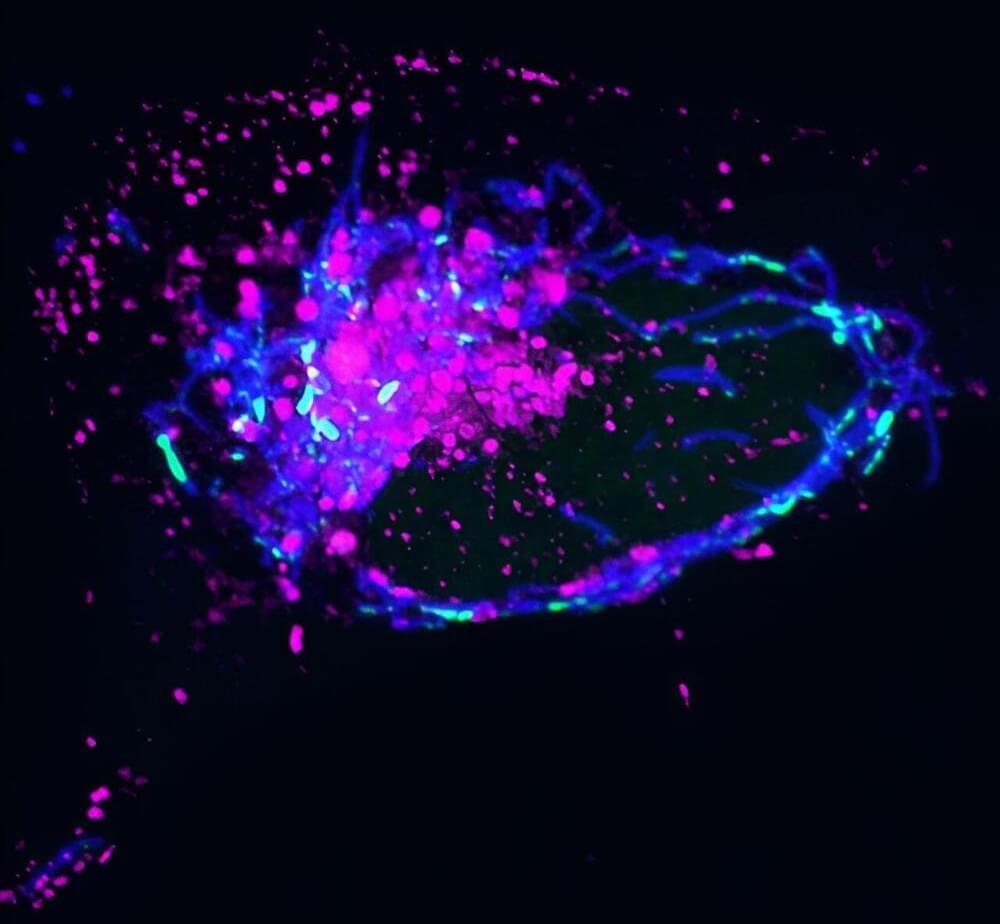
Cells in the human body contain power-generating mitochondria, each with their own mtDNA—a unique set of genetic instructions entirely separate from the cell’s nuclear DNA that mitochondria use to create life-giving energy. When mtDNA remains where it belongs (inside of mitochondria), it sustains both mitochondrial and cellular health—but when it goes where it doesn’t belong, it can initiate an immune response that promotes inflammation.
Now, Salk scientists and collaborators at UC San Diego have discovered a novel mechanism used to remove improperly functioning mtDNA from inside to outside the mitochondria. When this happens, the mtDNA gets flagged as foreign DNA and activates a cellular pathway normally used to promote inflammation to rid the cell of pathogens, like viruses.
The findings, published in Nature Cell Biology, offer many new targets for therapeutics to disrupt the inflammatory pathway and therefore mitigate inflammation during aging and diseases, like lupus or rheumatoid arthritis.




 We’ve updated our list of the best longevity experts on Twitter/X and added 8 new accounts, including Dr. Morgan Levine, Dr. Brad Stanfield, and the research journal Nature Aging!
We’ve updated our list of the best longevity experts on Twitter/X and added 8 new accounts, including Dr. Morgan Levine, Dr. Brad Stanfield, and the research journal Nature Aging!