Feb 2, 2017
Viral protein transforms as it measures out DNA
Posted by Karen Hurst in categories: biotech/medical, genetics, particle physics
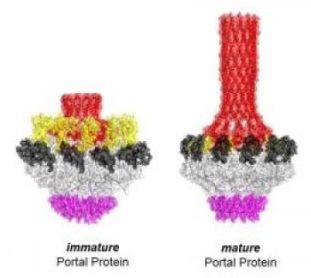
To generate swarms of new viral particles, a virus hijacks a cell into producing masses of self-assembling cages that are then loaded with the genetic blueprint for the next infection. But the picture of how that DNA is loaded into those viral cages, or capsids, was blurry, especially for two of the most common types of DNA virus on earth, bacterial viruses and human herpesvirus. Jefferson researchers pieced together the three-dimensional atomic structure of a doughnut-shaped protein that acts like a door or ‘portal’ for the DNA to get in and out of the capsid, and have now discovered that this protein begins to transform its structure when it comes into contact with DNA. Their work published in Nature Communications.
“Researchers thought that the portal protein acts as an inert passageway for DNA,” says senior author Gino Cingolani, Ph.D., a Professor in the Department of Biochemistry and Molecular Biology at Thomas Jefferson University and researcher at the Sidney Kimmel Cancer Center. “We have shown that the portal is much more like a sensor that essentially helps measure out an appropriate length of DNA for each capsid particle, ensuring faithful production of new viral particles.”
The finding solves a longstanding puzzle in the field, and reveals a potential drug target for one of the most common human viral pathogens, herpesviruses, which is responsible for diseases such as chicken pox, mononucleosis, lymphomas and Kaposi sarcoma.