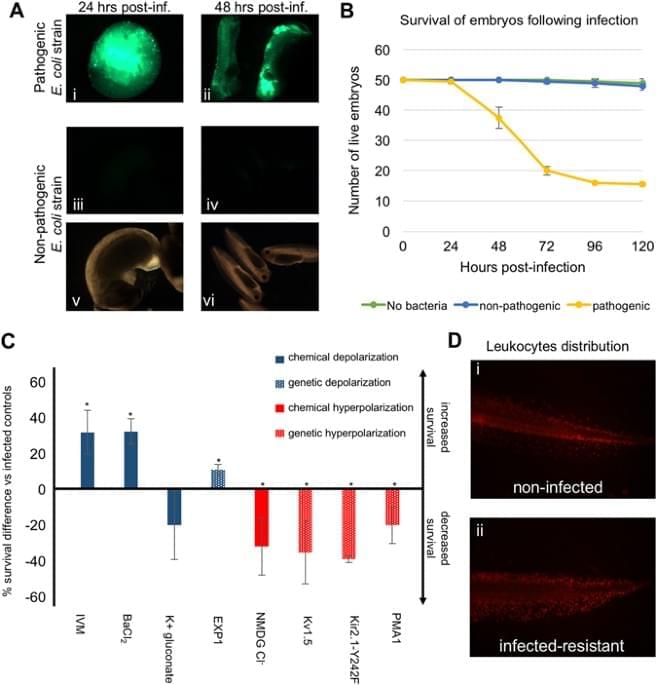
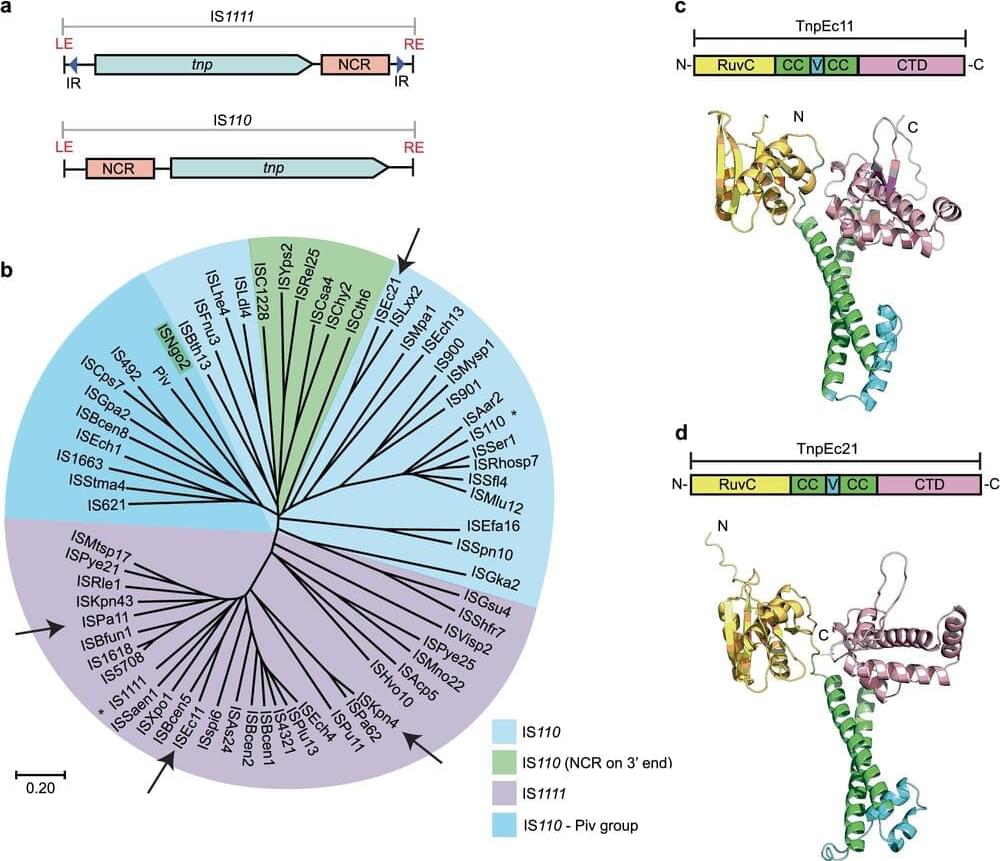
Jul 26, 2024
New Technology to Control the Brain Using Magnetic Fields Developed
Posted by Saúl Morales Rodriguéz in categories: biotech/medical, computing, genetics, nanotechnology, neuroscience
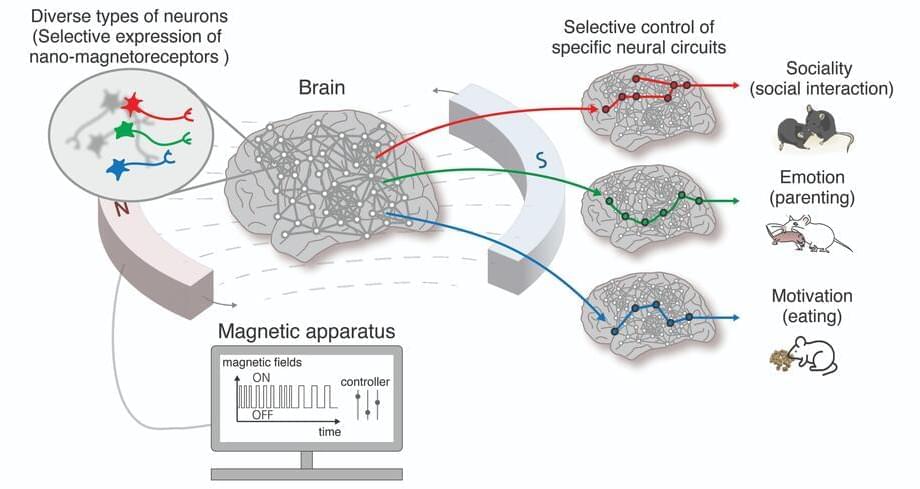
Nano-MIND Technology for Wireless Control of Brain Circuits with Potential to Modulate Emotions, Social Behaviors, and Appetite.
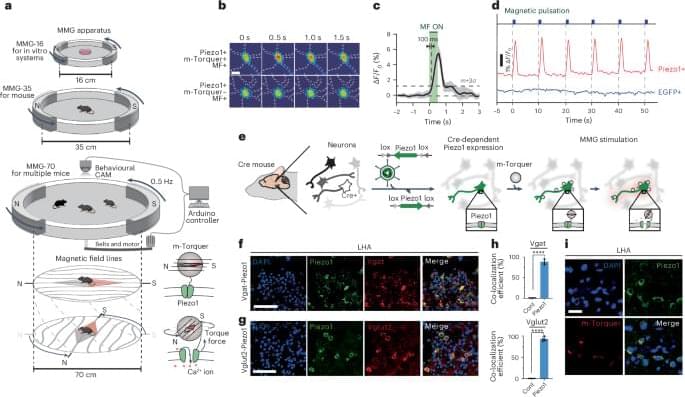
Researchers at the Center for Nanomedicine within the Institute for Basic Science (IBS) and Yonsei University in South Korea have unveiled a groundbreaking technology that can manipulate specific regions of the brain using magnetic fields, potentially unlocking the secrets of high-level brain functions such as cognition, emotion, and motivation. The team has developed the world’s first Nano-MIND (Magnetogenetic Interface for NeuroDynamics) technology, which allows for wireless, remote, and precise modulation of specific deep brain neural circuits using magnetism.
The human brain contains over 100 billion neurons interconnected in a complex network. Controlling the neural circuits is crucial for understanding higher brain functions like cognition, emotion, and social behavior, as well as identifying the causes of various brain disorders. Novel technology to control brain functions also has implications for advancing brain-computer interfaces (BCIs), such as those being developed by Neuralink, which aim to enable control of external devices through thought alone.
Continue reading “New Technology to Control the Brain Using Magnetic Fields Developed” »