Archive for the ‘computing’ category: Page 267
Mar 13, 2023
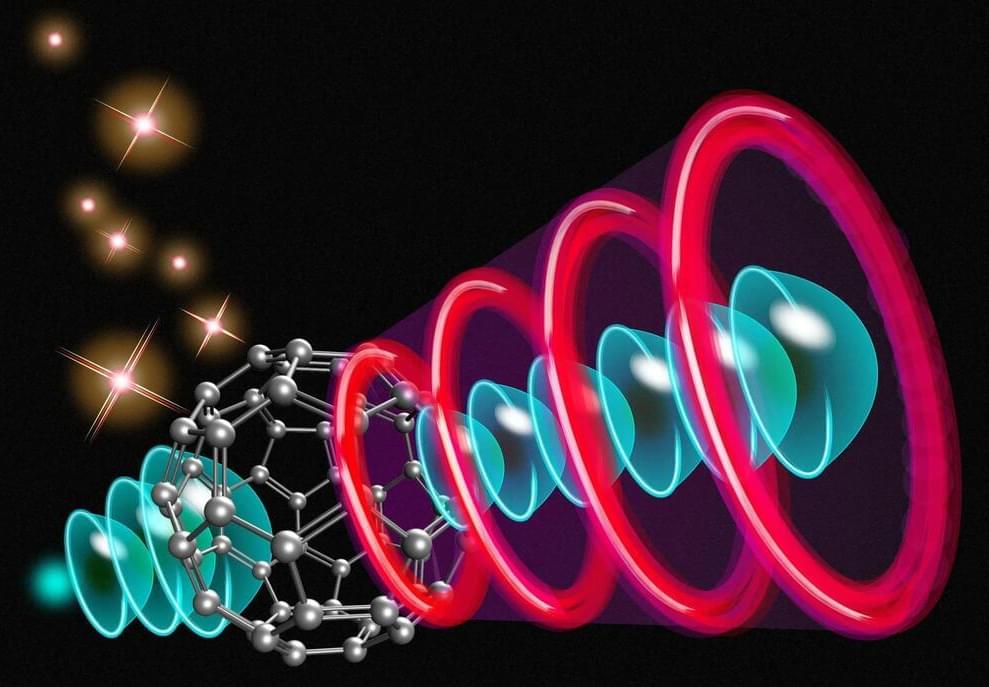
Up to 1,000,000 Times Faster: A Switch Made From a Single Molecule
Posted by Jose Ruben Rodriguez Fuentes in categories: computing, physics
An international team of researchers, including those from the University of Tokyo’s Institute for Solid State Physics, has made a groundbreaking discovery. They have successfully demonstrated the use of a single molecule named fullerene as a switch, similar to a transistor. The team achieved this by employing a precisely calibrated laser pulse, which allowed them to control the path of an incoming electron in a predictable manner.
The switching process enabled by fullerene molecules can be significantly faster than the switches used in microchips, with a speed increase of three to six orders of magnitude, depending on the laser pulses utilized. The use of fullerene switches in a network could result in the creation of a computer with capabilities beyond what is currently achievable with electronic transistors. Additionally, they have the potential to revolutionize microscopic imaging devices by providing unprecedented levels of resolution.
Over 70 years ago, physicists discovered that molecules emit electrons in the presence of electric fields, and later on, certain wavelengths of light. The electron emissions created patterns that enticed curiosity but eluded explanation. But this has changed thanks to a new theoretical analysis, the ramification of which could not only lead to new high-tech applications but also improve our ability to scrutinize the physical world itself.
Mar 13, 2023
Nokia Magic Max, Nokia is About to Reclaim its Crown with this Ultimate Flagship
Posted by Raphael Ramos in categories: computing, mobile phones

Reports say that the Nokia Magic Max will come in three different memory configurations. We will have 8GB, 12GB and 16GB of RAM with 256GB and 512GB storage options. It will launch with Android 13 out of the box with Snapdragon 8 Gen 2 SoC under the hood. We may also see a 6.7-inch AMOLED display with 120Hz refresh rate on the device. Corning Gorilla Glass 7 protection could be on the display of the upcoming flagship device from Nokia.
The device will feature a triple camera setup on the back with 144MP main sensor, 64MP ultrawide and 48MP Telephoto lens. Rumors have suggested a massive 7950mAh battery which can also charge from 0 to 100 within a few minutes, thanks to the 180W fast charger.
Mar 13, 2023
Uneven Circuit Aging Becoming A Bigger Problem
Posted by Saúl Morales Rodriguéz in categories: computing, engineering, life extension
The industry is gaining ground in understanding how aging affects reliability, but more variables make it harder to fix.
Circuit aging is emerging as a first-order design challenge as engineering teams look for new ways to improve reliability and ensure the functionality of chips throughout their expected lifetimes.
The need for reliability is obvious in data centers and automobiles, where a chip failure could result in downtime or injury. It also is increasingly important in mobile and consumer electronics, which are being used for applications such as in-home health monitoring or for navigation, and where the cost of the devices has been steadily rising. But aging also needs to be assessed in the context of variation models from the foundries, different use cases that may stress various components in different ways, and different power and thermal profiles, all of which makes it harder to accurately predict how a chip will behave over time.
Mar 13, 2023
Designing for Data Flow
Posted by Saúl Morales Rodriguéz in categories: computing, materials
Processing more data in more places while minimizing its movement becomes a requirement and a challenge.
Movement and management of data inside and outside of chips is becoming a central theme for a growing number of electronic systems, and a huge challenge for all of them.
Entirely new architectures and techniques are being developed to reduce the movement of data and to accomplish more per compute cycle, and to speed the transfer of data between various components on a chip and between chips in a package. Alongside of that, new materials are being developed to increase electron mobility and to reduce resistance and capacitance.
Mar 12, 2023
Immersive Virtual Reality From The Humble Webcam
Posted by Dan Breeden in categories: computing, information science, space, virtual reality
[Russ Maschmeyer] and Spatial Commerce Projects developed WonkaVision to demonstrate how 3D eye tracking from a single webcam can support rendering a graphical virtual reality (VR) display with realistic depth and space. Spatial Commerce Projects is a Shopify lab working to provide concepts, prototypes, and tools to explore the crossroads of spatial computing and commerce.
The graphical output provides a real sense of depth and three-dimensional space using an optical illusion that reacts to the viewer’s eye position. The eye position is used to render view-dependent images. The computer screen is made to feel like a window into a realistic 3D virtual space where objects beyond the window appear to have depth and objects before the window appear to project out into the space in front of the screen. The resulting experience is like a 3D view into a virtual space. The downside is that the experience only works for one viewer.
Eye tracking is performed using Google’s MediaPipe Iris library, which relies on the fact that the iris diameter of the human eye is almost exactly 11.7 mm for most humans. Computer vision algorithms in the library use this geometrical fact to efficiently locate and track human irises with high accuracy.
Mar 12, 2023
Aluminum-based low-loss interconnects for superconducting quantum processors
Posted by Saúl Morales Rodriguéz in categories: computing, quantum physics
Quantum processors are computing systems that process information and perform computations by exploiting quantum mechanical phenomena. These systems could significantly outperform conventional processors on certain tasks, both in terms of speed and computational capabilities.
While engineers have developed several promising quantum computing systems over the past decade or so, scaling these systems and ensuring that they can be deployed on a large-scale remains an ongoing challenge. One proposed strategy to increase the scalability of quantum processors entails the creation of modular systems containing multiple smaller quantum modules, which can be individually calibrated and then arranged into a bigger architecture. This, however, would require suitable and effective interconnects (i.e., devices for connecting these smaller modules).
Researchers at the Southern University of Science and Technology, the International Quantum Academy and other institutes in China have recently developed low-loss interconnects for linking the individual modules in modular superconducting quantum processors. These interconnects, introduced in Nature Electronics, are based on pure aluminum cables and on-chip impendence transformers.
Mar 10, 2023
The Future of Computing Includes Biology: AI Computers Powered by Human Brain Cells
Posted by Jose Ruben Rodriguez Fuentes in categories: biotech/medical, computing, neuroscience

The future of computing includes biology says an international team of scientists.
The time has come to create a new kind of computer, say researchers from John Hopkins University together with Dr. Brett Kagan, chief scientist at Cortical Labs in Melbourne, who recently led development of the DishBrain project, in which human cells in a petri dish learned to play Pong.
Mar 10, 2023
This Giant Scorpion Gaming Chair is a Zero-Gravity Computer Workstation That Cocoons You
Posted by Omuterema Akhahenda in categories: computing, entertainment
Working from home has many of us wondering how we can make this new experience more comfortable and accommodating. lately we’ve seen brands like established & sons collaborate with french designers erwan and ronan bouroullec to create flexible pieces of furniture that really work for these changing times. but this new chair got us both excited and confused as we can’t decide if it’s genius or just borderline crazy. developed by cluvens, the cluvens IW-SK zero-gravity esports gaming chair boast a scorpion shape that cocoons you — if that’s what you like.
Mar 9, 2023
‘Revolutionary’ blue crystal resurrects hope of room temperature superconductivity
Posted by Kelvin Dafiaghor in categories: computing, physics
Has the quest for room temperature superconductivity finally succeeded? Researchers at the University of Rochester (U of R), who previously were forced to retract a controversial claim of room temperature superconductivity at high pressures, are back with an even more spectacular claim. This week in they report a new material that superconducts at room temperature—and not much more than ambient pressures.
“If this is correct, it’s completely revolutionary,” says James Hamlin, a physicist at the University of Florida who was not involved with the work. A room temperature superconductor would usher in a century-long dream. Existing superconductors require expensive and bulky chilling systems to conduct electricity frictionlessly, but room temperature materials could lead to hyperefficient electricity grids and computer chips, as well as the ultrapowerful magnets needed for levitating trains and fusion power.
But given the U of R group’s recent retraction, many physicists won’t be easily convinced. “I think they will have to do some real work and be really open for people to believe it,” Hamlin says. Jorge Hirsch, a physicist at the University of California, San Diego, and a vociferous critic of the earlier work, is even more blunt. “I doubt [the new result], because I don’t trust these authors.”